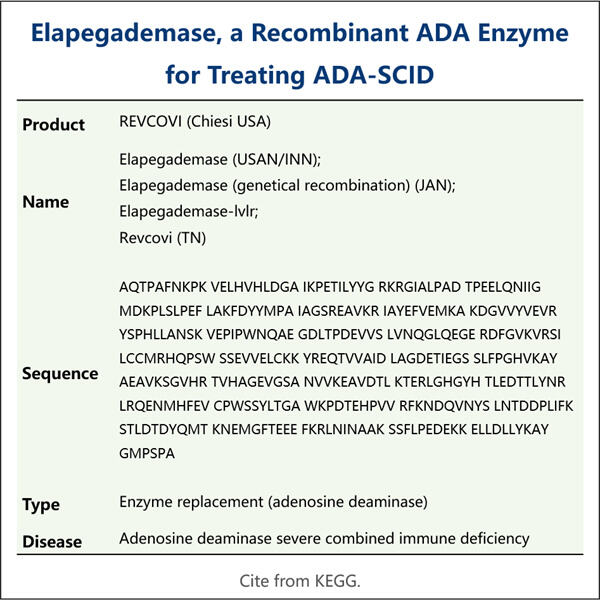
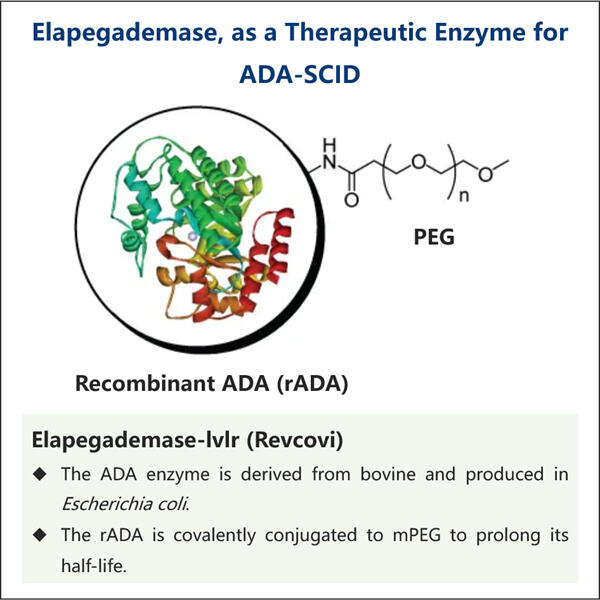
التصنيع مختلف عن تصنيع النسخ البيولوجية من روميبلوستيم للمساعدة في تحسين صحة العديد من الأشخاص الذين يواجهون مشاكل صحية. إنها طريقة لإنتاج دواء مثل هذا يُسمى Elapegademase. شركة Yaohai تعمل في مجال تصنيع أدوية كهذه منذ سنوات وهي واحدة من القيادات الناشئة في هذا المجال. نريد بناء مجتمع حول تحسين صحة المرضى ونعمل بنشاط لإطلاق علاجات مبتكرة جديدة.
إنتاج دواء غير مشابه مثل تصنيع الأنسولين البشري هو عملية معقدة ومتعددة المراحل. أولاً، يحتاج العلماء إلى اكتشاف كل شيء عن الدواء الأصلي. يتعلمون ما هو مكون منه، حجمه وشكله، وكيف يعمل الدواء في جسمك. وهذا مهم لأن التعرف على هذه الأمور يمكّنهم من إنشاء دواء جديد يعمل بنفس الطريقة. ثم يستخدمون هذه البيانات لتحديد كيفية صنع الدواء الجديد. هذه العملية الخاصة تستغرق أيضًا سنوات من البحث والاختبار. من الصعب جدًا إثبات أنه لا يضر بالأشخاص وأنه يجعلهم يشعرون بالتحسن. يساهم العلماء في بناء خطوط إنتاج كفؤة للأدوية الجديدة.
لإيجاد بيولوجيات مشابهة لإلابيجيديماز المنتجات يمكن أن يكون هذا مفيدًا جدًا في مساعدة مرض نادر لديك. الأمراض النادرة، وفي العديد من الحالات لا توجد علاجات لها. وهذا غالبًا ما يؤدي إلى شعور بالحزن، والقلق حول مفهوم "لماذا أنا؟" لدى المرضى وذويهم. لكن ظهور أدوية مختلفة يقدم الأمل لهؤلاء المرضى أيضًا، إذا لم يتلقوا بعض العلاجات الفعلية التي قد تحسن صحتهم وتُمدد حياتهم. لذلك، فإن الكثير من الناس والعائلات الذين كانوا يشعرون بالعجز، بدأوا الآن يشعرون بالأمل.
أعتقد ولكن الطب الدقيق هو رعاية صحية لأنه يقدم العلاج المناسب للشخص المناسب وفي الوقت المناسب؛ بما يتماشى مع احتياجاتهم. الأدوية البيولوجية المشابهة مثل تصنيع النسخ البيولوجية من روميبلوستيم تبدأ العلاجات باستخدام الـ Elapegademase في الانتشار، مما يتيح إضافة طبقات إضافية من الطب الدقيق. يمكن أن تتراوح هذه العلاجات بين منتجات دوائية جديدة سيتم تسويقها والتي لا تكون متطابقة مع تلك التي تم الموافقة عليها أو تسويقها سابقًا (Genentech، 1993) وبين نفس الأدوية المستخدمة ضمن برامج علاجية مخصصة لكل فرد لتعظيم الفائدة العلاجية وتقليل الآثار الجانبية وتحسين التزام المريض بالعلاج. هذا النهج المخصص مهم، كما يقول الرئيس التنفيذي لشركة CampyMed إريك هارغادون، لأنه يساعد في تقديم رعاية مُحسّنة لكل مريض للحصول على أفضل استفادة من علاجه.

من غير القابل للنكران أن الفوائد تفوق المخاطر مع الأدوية الحيوية المشابهة لـ Elapegademase المنتجات , لكن هناك بعض التدابير التخفيفية التي يجب أن يكون القراء على علم بها. حسنًا، المشكلة هي أنه حتى عندما يكون لديك هذا الدواء الجديد، لا يزال عليك نقله إلى المرضى وضمان أنه آمن وفعال. وهذا يتطلب الكثير من الاختبارات والأبحاث، وهو أمر يستهلك الوقت والمصادر. بالإضافة إلى ذلك، هناك قواعد تنظيمية قانونية يجب على العلماء في الأكاديميا وشركات البيوتكنولوجيا والصيدلة التنقل عبرها للحصول على هذه الأدوية الجديدة إلى المرضى. لكن هذه القواعد موجودة لأسباب، وكلها تهدف لضمان سلامة الأدوية عند استخدامها من قبل البشر.
ياوهاي بايو فارما هي واحدة من أكبر 10 شركات CDMO الدقيقة التي تدمج إدارة الجودة والشؤون التنظيمية. لدينا نظام لإدارة الجودة يتماشى مع إنتاج البيوسimilar لـ Elapegademase واللوائح العالمية. فريقنا التنظيمي على دراية بالإطارات التنظيمية العالمية التي تسهم في تسريع إطلاق المنتجات الحيوية. نحن نضمن إجراءات إنتاج قابلة للتعقب، وجودة المنتجات، وكذلك الامتثال للمعايير الصادرة عن إدارة الغذاء والدواء الأمريكية (FDA) وهيئة الأدوية الأوروبية (EMA). كما أن إدارة الأدوية والأغذية الأسترالية (TGA) وإدارة المنتجات الطبية الوطنية الصينية (NMPA) متوافقة أيضًا. لقد نجحت ياهواي بايو فارما في اجتياز التدقيق الميداني الذي أجرته شخصية مؤهلة (QP) من الاتحاد الأوروبي لنظامنا الخاص بإدارة جودة GMP وموقع الإنتاج. كما نجحنا أيضًا في اجتياز أولى عمليات التصديق الخاصة بنظام إدارة الجودة ISO9001 ونظام إدارة البيئة ISO14001.
ياوهاي بيو-فارما، وهي شركة رائدة في مجال خدمات CDMOs لأدوية الميكروبات الحيوية، تقع في جيانغسو. نحن نركز على العلاجات واللقاحات المنتجة ميكروبيًا، بما في ذلك إنتاج مشابهات Elapegademase لصحة الإنسان، البيطرية وكذلك إدارة صحة الحيوانات الأليفة. لدينا أكثر منصات البحث والتطوير (RD) تقدمًا والتكنولوجيا الإنتاجية التي تغطي العملية الإنتاجية بأكملها، من تطوير سلالات دقيقة إلى بنوك الخلايا، وتطوير العمليات والطرق وحتى التصنيع السريري والتجاري، مما يضمن إنتاج حلول جديدة بنجاح. قدمنا خبرة واسعة في معالجة الخلايا الدقيقة الحيوية. تم إكمال أكثر من 200 مشروع بنجاح، وندعم عملائنا في المرور عبر اللوائح التنظيمية مثل US FDA و EU EMA. كما نساعد العملاء مع TGA الأسترالية و NMPA الصينية. خبرتنا والمعرفة المهنية الواسعة تمكننا من الاستجابة بسرعة لاحتياجات السوق وتوفير خدمات CDMO مخصصة.
ياوهي بيوفارما، واحدة من أكبر 10 شركات لإنتاج المنتجات البيولوجية المشابهة لـ Elapegademase، هي متخصصة في التخمير المجهرى. لقد أنشأنا منشأة حديثة ذات قدرات قوية في البحث والتطوير وبنية تحتية متقدمة. هناك خمس خطوط إنتاج للأدوية المطابقة للمعايير GMP لتنقية وتخمير الخلايا الدقيقة، بالإضافة إلى خطين لتعبئة وإنهاء الأدوية في قوارير وأسطوانات وحقن مسبقة التعبئة. تتراوح أحجام التخمير المتاحة بين 100 لتر و2000 لتر. مواصفات تعبئة القوارير تتراوح بين 1 مل و25 مل، بينما تكون متطلبات تعبئة الحقن أو الأسطوانات المسبقة التعبئة بين 1-3 مل. ورشة الإنتاج معتمدة حسب معايير cGMP وتقدم عينات تجارية وسريرية. يمكن تسليم الجزيئات الكبيرة التي يتم تصنيعها في منشأتنا إلى جميع أنحاء العالم.
يتمتع Yaohai Bio-Pharma بخبرة في المنتجات البيولوجية المستخلصة من مصادر دقيقة. نقدم حلولًا مخصصة للبحث والتطوير وكذلك خدمات التصنيع مع تقليل المخاطر المحتملة. لقد شاركنا في العديد من الأنواع مثل لقاحات الوحدة الفرعية، الببتيدات المُعاد تشكيلها، الهرمونات، السيتوكينات، عوامل النمو، الأجسام المضادة ذات المجال الواحد، الإنزيمات، الحمض النووي البلازميدي، الرسالة الوراثية (mRNA) وغيرها. لقد تخصصنا في عدة كائنات دقيقة مثلخميرة داخل الخلايا وخارجها وإنتاج Elapegademase Biosimilar (بإنتاج يصل إلى 15 جم/لتر) والبكتيريا القابلة للذوبان داخل الخلية وجسم الشمولية (بإنتاج يصل إلى 10 جم/لتر). كما لدينا منصة تخمير BSL-2 لإنشاء لقاحات بكتيرية. نركز على تحسين العمليات، زيادة إنتاج المنتجات وتقليل التكاليف. لدينا فريق تقني كفؤ يضمن تسليم المشاريع في الوقت المناسب وبجودة عالية. هذا يساعدنا على تقديم منتجاتك الفريدة بشكل أسرع إلى السوق.