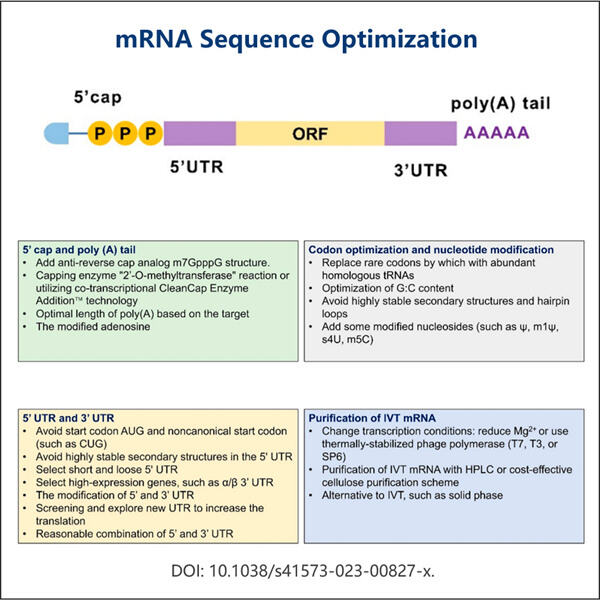
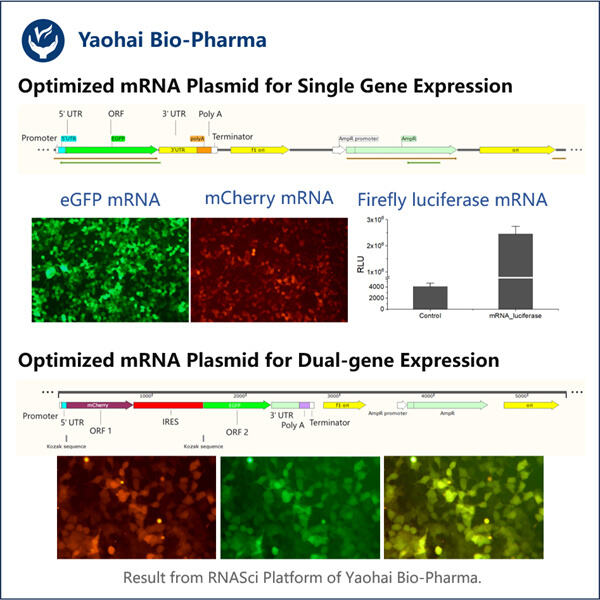
Pertama, mari kita mulai dengan mRNA. mRNA adalah singkatan dari messenger ribonucleic acid. Terdengar rumit, tetapi ini hanyalah pembantu khusus di dalam sel kita. mRNA adalah pembawa informasi berharga dari DNA ke berbagai bagian lainnya selain sel itu sendiri. DNA: Rencana Dasar untuk Tubuh Kita. Ini memiliki semua instruksi untuk mengarahkan sel kita tentang cara kerjanya. mRNA ini membawa informasi tersebut ke lokasi-lokasi di dalam sel di mana protein diproduksi.
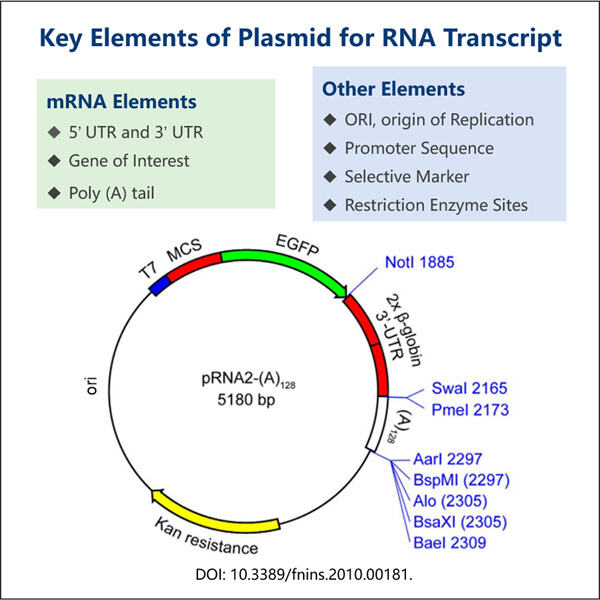
Selanjutnya, kita akan membahas Plasmid. Plasmid adalah molekul DNA ekstra kromosom yang kecil, berbentuk lingkaran, dua rantai, dan tidak termasuk dalam kromosom (DNA utama di sel kita) yang ditemukan secara alami dalam banyak bakteri, serta produk Yaohai seperti Produksi Kolagen Tipe III Rekombinan . Plasmid seperti kartu resep kecil. Bioteknolog biasanya menggunakannya untuk produksi protein. Bagian-bagian DNA ini disebut plasmid dan dengan menggunakan plasmid tersebut, kita bisa menyisipkan potongan DNA tertentu ke sel yang berbeda untuk menyatukan resep ideal guna membuat protein kita. Beberapa plasmid dapat memproduksi protein yang tidak ada dalam alam.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN