Kustomisasi, Efisiensi & Hemat Biaya
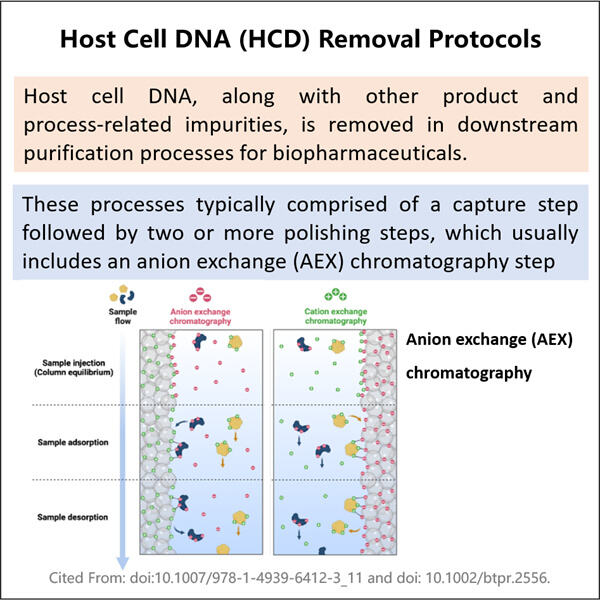
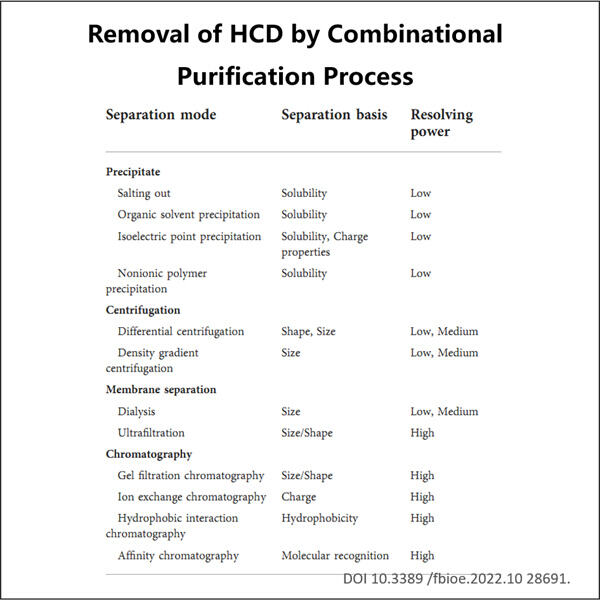
Yaohai Bio-Pharma berpengalaman dalam produk biologis yang berasal dari sumber mikroba. Kami menyediakan solusi RD yang disesuaikan dan manufaktur, sambil meminimalkan risiko potensial. Kami telah bekerja pada berbagai modali, termasuk vaksin subunit rekombinan, peptida hormon, sitokin faktor pertumbuhan, antibodi single-domain enzim, plasmid DNA berbagai mRNA, dan lainnya. Kami telah mengkhususkan diri pada beberapa mikroorganisme, termasuk Proses Penghilangan DNA Sel Tuan Rumah Ragi intraseluler dan ekstraseluler (hasil hingga 15g/L) dan bakteri larut intraseluler serta inklusi tubuh (hasil hingga 10g/L). Kami juga telah mendirikan platform fermentasi BSL-2 untuk membuat vaksin berbasis bakteri. Kami ahli dalam meningkatkan proses, meningkatkan hasil produk, dan menurunkan biaya produksi. Kami memiliki tim teknologi yang sangat efisien yang memastikan pengiriman proyek tepat waktu dan berkualitas tinggi. Hal ini memungkinkan kami membawa produk unik Anda lebih cepat ke pasar.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN