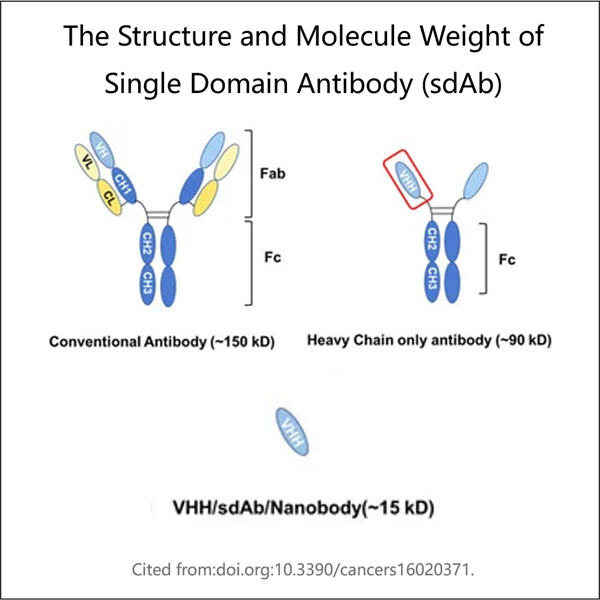
مصنع الأجسام المضادة البيونيكية. الأجسام المضادة هي عمال صغار في أجسادنا تساعد في محاربة الأمراض وإبقائنا بصحة جيدة. نوع واحد من الأجسام المضادة. للتحقق من قدرة ياوهاي على إنتاج الأجسام المضادة يتم استخدام نوع معين (Single Domain Antibody، sdAb). الأجسام المضادة الصغيرة هذه لديها قوة كبيرة ضد الأمراض.
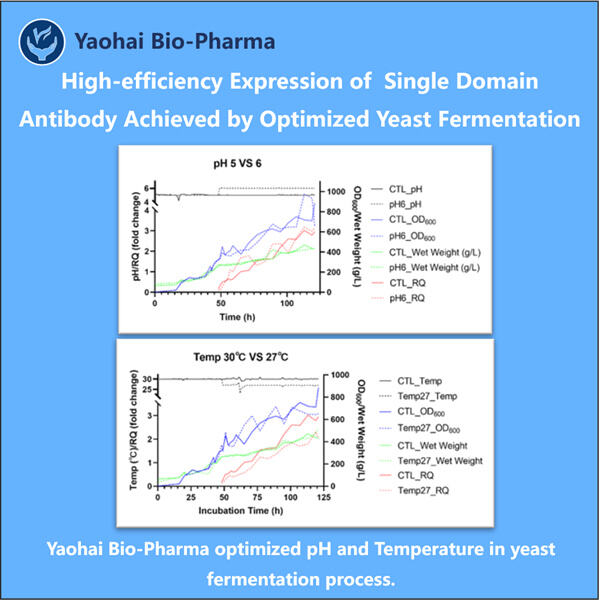
كان على ياوهاي وزملائه تطوير استراتيجية فريدة لثقافة الخلايا من أجل إنتاج كمية كبيرة من sdAbs. وبالتالي، هذه هي الخلايا التي تنتج sdAbs. بهذه الطريقة، يمكن لياوهاي إنتاج العديد من sdAbs لتكون متطابقة. هذا معلمة حرجة لأن sdAbs المتطابقة لديها إمكانات أكبر لمحاربة المسببات المرضية بشكل أكثر فعالية. كما يضمنون أن الخلايا مزودة بالكامل حتى تتمكن من النمو وإنتاج إنتاج VHH مضاد لـ vWF . هذه الرعاية والاهتمام تضمن أن sdAbs تكون فعالة للغاية في محاربة الأمراض.
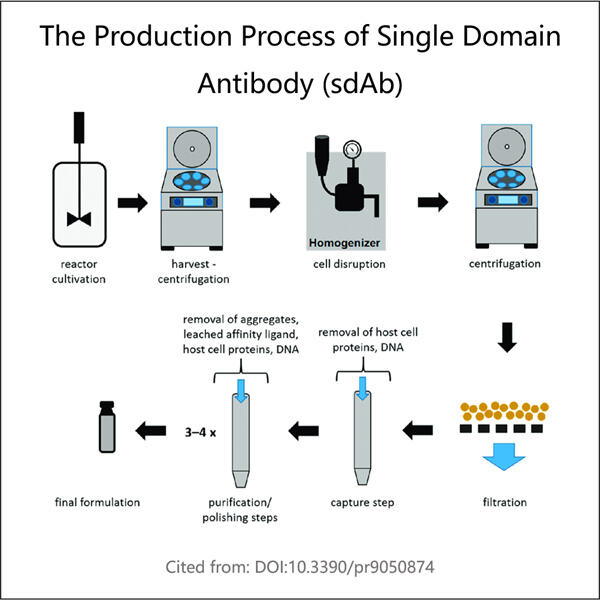
بعد أن تنتهي الخلايا من صنع sdAbs، يجب على ياوهاي استخراجها من الخلايا. يتم تحقيق ذلك باستخدام إجراء تنقية. هذه خطوة تنقية مهمة لضمان وجود sdAbs فقط في الدواء، وليس أي مواد خلوية متبقية. هذا مهم لأننا نرغب في أن يكون الدواء نقيًا وفعالًا.
قامت ياوهاي بتحسين تنقية sdAbs بطريقة سريعة وذكية. القدرة على تنقية sdAbs بسرعة باستخدام هذا الأسلوب هي أمر مهم. هذا مهم ببساطة لأنهم يمكنهم إنتاج العديد من sdAbs في وقت قصير. مما يعني أنهم يمكنهم إنتاج الدواء لعدد أكبر من الناس بشكل أسرع، حتى يشعر المزيد من الناس بالتحسن في وقت أقرب.
ياهاي وفريقه قاموا بإنشاء بعض الطرق الدقيقة للغاية لفحص sdAbs. ليس فقط أنها دقيقة للغاية، ولكن الأهم من ذلك أنها تساعد في ضمان سلامة sdAbs من خلال فهم ما سيتم وما لن يتم استهدافه في جسم الإنسان. هذا إنتاج VHH مضاد EGFR وفقًا لـ GMP سيضمن أن الدواء آمن وفعال لجميع الذين يحتاجون إليه.
هم حتى يبحثون عن أنواع الخلايا التي يمكنها إنتاج sdAbs بشكل أفضل مما تم اكتشافه في الفحص الأولي. هذا يساعدهم على تطوير المزيد من sdAbs التي يمكنها العمل بكفاءة في المجالات المستهدفة. هذا سيزيد من تنوع الخلايا التي يمكنهم فحصها لإنتاج sdAbs وتحسين عمليات التهجين لديهم لإنتاج sdAbs أكثر فعالية.
كم من الوقت يستمر تأثيرها؟ ثم، كيف يمكن لهذا الشخص الذي عملت معه جعلها مستقرة جدًا بحيث يكون لها عمر افتراضي طويل أيضًا. وأهم شيء هو الحفاظ على الدواء في مكان حيث يظل فعالًا لفترة أطول. والحفاظ إنتاج VHH مضاد HER3 وفق معايير GMP التخزين بعناية يعني أن المزيد من الناس يمكنهم الحصول على الدواء دون خوف من أنه لن يعمل بعد الآن.
تطوير عملية الأجسام المضادة أحادية المجال (sdAb) هو رائد في مجال CDMO البيولوجيات الدقيقة. تركيزنا كان دائمًا على اللقاحات والعلاجات المنتجة ميكروبيًا والمخصصة لإدارة الصحة البشرية، البيطرية، وكذلك صحة الحيوانات الأليفة. نحن نمتلك أكثر منصات RD تقدمية وتقنيات التصنيع التي تغطي العملية بأكملها، بدءًا من تطوير سلالات دقيقة وبنوك الخلايا، إلى تطوير العمليات والطرق، وحتى الإنتاج السريري والتجاري مما يضمن تسليم حلول مبتكرة بنجاح. مع مرور الوقت، جمعنا خبرة واسعة في معالجة البيولوجيات القائمة على الميكروبات. تم إكمال أكثر من 200 مشروع بنجاح، ونساعد عملائنا على الامتثال للوائح مثل US FDA و EU EMA. كما نساعد في التنقل عبر لوائح Australia TGA و China NMPA. يسمح لنا خبرتنا المهنية وخبرتنا الواسعة بالاستجابة بسرعة لاحتياجات السوق وتقديم خدمات CDMO مخصصة.
ياوهاي بيوفارما هي واحدة من أكبر 10 شركات CDMO الدقيقة التي تدمج إدارة الجودة والقضايا التنظيمية. لقد طورنا نظامًا قويًا لإدارة الجودة يتماشى مع معايير GMP الحالية واللوائح العالمية. فريقنا التنظيمي لديه فهم عميق للإطارات التنظيمية العالمية. هذا يمكّننا من تسريع إطلاق المنتجات البيولوجية. نحن نضمن عمليات إنتاج قابلة للتتبع وكذلك منتجات ذات جودة عالية ومتصلة بالمبادئ التوجيهية للهيئة الأمريكية للأغذية والعقاقير (FDA) وهيئة الأدوية الأوروبية (EMA). تطوير عملية الأجسام المضادة أحادية المجال (sdAb) وإدارة الهيئة الوطنية للإدارة الدوائية في الصين (NMPA) راضية أيضًا. تمكنت ياوهاي بيوفارما بنجاح من اجتياز التدقيق الميداني الذي أجراه شخص مؤهل معتمد من الاتحاد الأوروبي (QP) لمراجعة نظام GMP ومرافق الإنتاج لدينا. كما مررنا أيضًا بعمليات التصديق الأولى لنظام إدارة الجودة ISO9001 ونظام إدارة البيئة ISO14001.
ياوهاي بيولوجي فارما متخصصة في تطوير عمليات الأجسام المضادة ذات المجال الواحد (sdAb) المستمدة من الكائنات الدقيقة. نقدم حلول بحث وتطوير مخصصة وكذلك حلول تصنيعية مع تقليل المخاطر إلى الحد الأدنى. لقد شاركنا في العديد من النماذج مثل اللقاحات الفرعية рекомбинانت، الهرمونات الببتيدية، السيتوكينات عوامل النمو، الأجسام المضادة ذات المجال الواحد، الإنزيمات، الحمض النووي البلازميدي، الرنا المرسال، وغيرها. نحن خبراء في عدة مستضيفات دقيقة مثل الخميرة داخل الخلايا وخارجها (إنتاج يصل إلى 15 جراماً لكل لتر)، إفراز البيروبلسم البكتيري بالإضافة إلى الجسيمات الداخلية القابلة للذوبان (إنتاج يصل إلى 10 جرامات/لتر). بالإضافة إلى ذلك، قمنا بتطوير منصة تخمير بكتيري المستوى BSL-2 لتطوير لقاحات بكتيرية. لدينا سجل حافل في تحسين العمليات الإنتاجية، مما يؤدي إلى زيادة الإنتاج وتقليل التكاليف. ومع فريق تقني كفؤ للغاية، نضمن تسليم المشاريع بشكل سريع وموثوق، مما يساعد على إدخال منتجاتكم إلى السوق بشكل أسرع.
ياوهي بيو-فارما، وهي شركة متخصصة في تطوير عمليات الأجسام المضادة أحادية المجال (sdAb) لتصنيع المنتجات البيولوجية، لديها خبرة في التخمير المجهرية. لقد بنينا مصنعًا حديثًا مزودًا بمعدات حديثة وقدرات قوية في البحث والتطوير والتصنيع. هناك خمس خطوط إنتاج مواد دوائية تتوافق مع معايير GMP للتنقية والتخمير المجهرية، بالإضافة إلى خطين لتعبئة الأنبوب وزجاجات الكرباج والحقن المسبقة التعبئة. تتراوح أحجام التخمير المتاحة من 100 لتر إلى 500 لتر، 1000 لتر، وحتى 2000 لتر. تتباين أحجام التعبئة بين 1 مل و25 مل. يتم تعبئة الحقن أو زجاجات الكرباج المسبقة التعبئة بحجم يتراوح بين 1-3 مل. يضمن مصنع الإنتاج الخاص بنا الذي يتوافق مع cGMP توفر عينات سريرية مستقرة ومنتجات تجارية. يمكن توفير الجزيئات الكبيرة التي تنتج في مصنعنا للتسليم الدولي.