لا يمكننا الانتظار لعرض كيفية تصنيع منتجنا المطابق لمعايير GMP، VHH مضاد لـ EGFR! يتم ذلك لضمان أن أي شيء تقوم بإنتاجه يكون آمنًا ومتوافقًا مع معايير الجودة. نأمل أن يتم استخدام هذه الطريقة الجديدة لتطوير علاجات ضد السرطان، وهو مرض يهدد الحياة. طريقة إنتاج يوهاي GMP Anti-PD-1PD-L1 VHH ليست فقط سريعة، ولكنها أيضًا تتبع جميع الإرشادات المتعلقة بالجودة اللازمة لحماية المرضى وتحسين نتائجهم.
إحدى الخطوات الرئيسية في علاج السرطان هي إنتاج anti-EGFR VHH. يحتاج الأطباء إلى هذا المنتج لعلاج المرضى بشكل أفضل. يمكن لياوهاي الإنتاج بشكل أسرع وأكثر كفاءة وحققوا معايير جودة عالية باستخدام طرق GMP. هذه التطورات تعني إنقاذ المزيد من الأرواح من خلال المساعدة في البحث عن علاجات أفضل للسرطان. نعمل بجد حتى يتمكن العديد من الناس من العيش بسعادة وأمل أكبر من خلال مساهمتنا في محاربة السرطان عبر إنتاجنا.
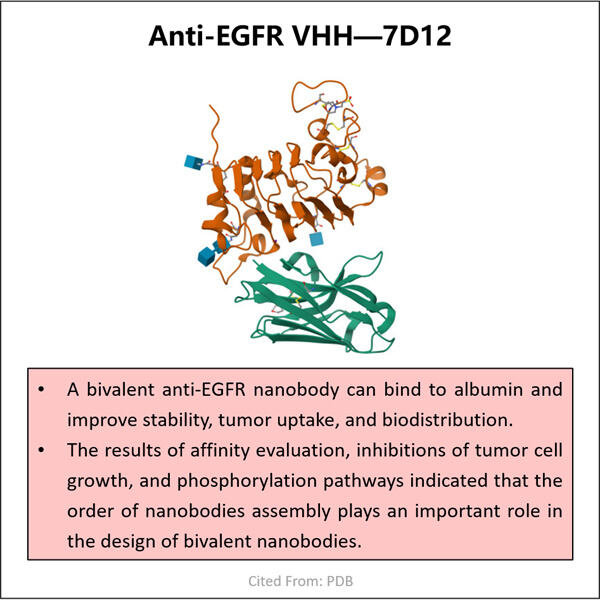
الشيء نفسه ينطبق عند إعداد VHH مضاد لـ EGFR. ونحن في ياوهاي ندرك أن المرضى يضعون ثقتهم فينا لتوفير منتجات آمنة وموثوقة. لهذا السبب نضمن الالتزام بجميع المعايير GMP المعمول بها أثناء التصنيع. هذه مسؤولية كبيرة بالنسبة لنا لأننا حقًا نريد إنشاء منتجات استثنائية. الشيء الذي يميزنا عن منافسينا هو جودة منتجاتنا، ونفعل كل ما في وسعنا للحفاظ على هذه المعايير بالدفعة.
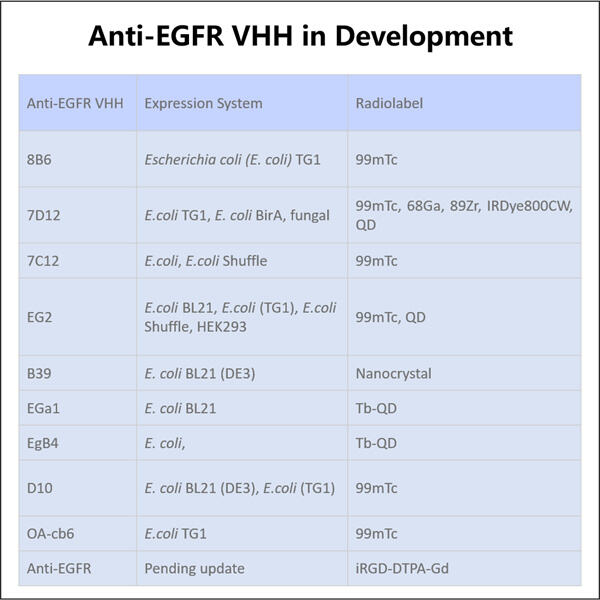
إحدى النقاط الرئيسية في مختبرنا في ياوهاي هي إنشاء ياوهاي إنتاج VHH مضاد HER3 وفق معايير GMP مع شروط GMP الصارمة. عملية يمكننا أن نفتخر بأنها لا تلتزم بجميع قواعد الجودة فقط، بل إنها أيضًا بسيطة وكفوءة. هذا يمكّننا من تصنيع منتجاتنا بطريقة أكثر استدامة مع الحفاظ على الجودة. نحن نطمح إلى تحسين طرق الإنتاج الخاصة بنا من أجل تحقيق فائدة للمريض النهائي.
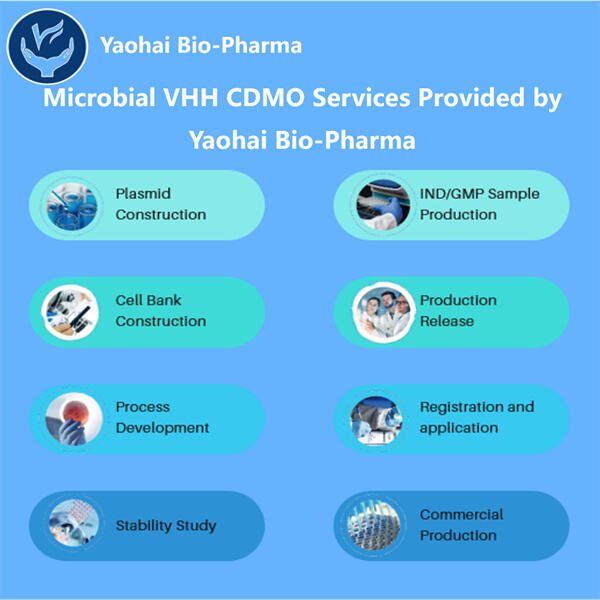
إنتاج الأدوية البيولوجية الدقيقة أمر حساس للغاية، ولأسباب عديدة تتعلق بالقواعد واللوائح المهمة العديدة. يجب أن نلتزم بالبروتوكولات القياسية حتى يحصل المرضى على المساعدة الأكثر دقة. يوهاي ملتزم بتقديم يوهاي إنتاج VHH مضاد CD8 وفقًا لنظام GMP . هذا الالتزام يجعلنا شريكًا فريدًا لإنتاج الأدوية البيولوجية الدقيقة، حيث يظل تركيزنا ثابتًا على سلامة المريض وجودة المنتج.
ياوهاي بيو-فارما، واحدة من أكبر 10 مصنعين للمنتجات البيولوجية، تخصصت في التخمير المجهرى. لقد بنينا منشأة حديثة ذات قدرات بحث وتطوير قوية ومعدات متقدمة. لدينا خمس خطوط لإنتاج المواد الدوائية التي تتوافق مع متطلبات GMP للتخمير والتنقية الميكروبية. كما أن لدينا خطين آليين لتعبئة الأدوية في القوارير والأمبولات والمحاقن الجاهزة. تتراوح أحجام التخمير المتاحة بين إنتاج VHH مضاد EGFR وفقًا لمعايير GMP وحتى 2000 لتر. فيما تتراوح مواصفات تعبئة القوارير من 1 مل إلى 25 مل، بينما تتراوح مواصفات تعبئة المحاقن أو الأنابيب الجاهزة بين حوالي 1-3 مل. منشأتنا المنتجة conforme لمعايير cGMP تضمن توفير العينات السريرية بشكل مستمر وكذلك المنتجات التجارية. مصنعنا ينتج جزيئات كبيرة يتم شحنها حول العالم.
لدى Yaohai Bio-Pharma خبرة في تصنيع الأدوية البيولوجية المصنوعة من المجهريات. نقدم حلول RD مخصصة وخدمات التصنيع مع تقليل المخاطر إلى الحد الأدنى. عملنا مع أنماط متنوعة مثل اللقاحات الفرعية рекомбинانت، الببتيدات، الهرمونات، السيتوكينات، عوامل النمو، الأجسام المضادة أحادية المجال، الإنزيمات، الحمض النووي البلازميدي، mRNA وغيرها. تخصصنا في عدة مجهريات مثلخميرة السكر الإفراز الخلوي والخلوي الداخلي (الإنتاج يصل إلى 15 جم/لتر)، البكتيريا الذوبان الخلوي والجسيمات الشاملة (الإنتاج يصل إلى 10 جم/لتر). كما قمنا بإنشاء نظام تخمير BSL-2 لإنتاج GMP Anti-EGFR VHH واللقاحات. نحن خبراء في تحسين عمليات الإنتاج، زيادة الإنتاج وتقليل التكاليف. لدينا فريق تقني كفؤ للغاية يضمن تسليم المشاريع في الوقت المحدد وبجودة عالية. هذا يسمح لنا بتقديم منتجاتك الفريدة بشكل أسرع إلى السوق.
إنتاج GMP Anti-EGFR VHH هو رائد في مجال CDMO البيولوجي المجهرى. تركيزنا كان دائمًا على اللقاحات والعلاجات المُنتجة ميكروبيًا والمُناسبة لإدارة الصحة البشرية، والبيطرية، وكذلك صحة الحيوانات الأليفة. لدينا أكثر منصات RD تقدمًا وتقنيات التصنيع التي تغطي العملية بأكملها بدءًا من تطوير سلالات دقيقة وبنوك الخلايا، إلى تطوير العمليات والطرق، وحتى الإنتاج التجاري والسريري مما يضمن تسليم حلول مبتكرة بنجاح. مع مرور الوقت، جمعنا معرفة واسعة حول معالجة البيولوجيا القائمة على الكائنات الدقيقة. تم إكمال أكثر من 200 مشروع بنجاح، ونساعد عملائنا على الامتثال للوائح مثل US FDA و EU EMA. نساعد أيضًا في التنقل عبر لوائح Australia TGA و China NMPA. يسمح لنا خبرتنا المهنية وخبرتنا الواسعة بالاستجابة السريعة لاحتياجات السوق وتقديم خدمات CDMO مخصصة.
ياوهاي بيوفارما، واحدة من أكبر 10 شركات CDMO المجهرية، تدمج الأمور المتعلقة بالجودة والتنظيم. لدينا نظام جودة متوافق تمامًا مع المعايير الحالية لـ GMP وكذلك اللوائح الدولية. فريق خبراء التنظيم لدينا لديه فهم عميق للإطارات التنظيمية العالمية. هذا يمكّننا من تسريع إطلاق المنتجات البيولوجية. نحن قادرون على ضمان إجراءات إنتاج قابلة للتتبع ومنتجات ذات جودة عالية تتماشى مع اللوائح الصادرة عن US FDA، GMP Anti-EGFR VHH Production، Australia TGA، و China NMPA. لقد نجحت ياوهاي بيوفارما في اجتياز التدقيق الميداني الذي أجرته شخصية مؤهلة (QP) من الاتحاد الأوروبي لنظام الجودة الخاص بنا وموقع الإنتاج. كما مررنا أيضًا بمراحل التدقيق الأولية لنظام إدارة الجودة ISO9001 ونظام إدارة البيئة ISO14001.