
Microbial CRDMO Solutions, Accelerated with Precision
We are dedicated to delivering comprehensive end-to-end CRDMO solutions for global clients
Previous
Next
Who we are
Global Leader in Microbial CRDMO
Yaohai Bio-Pharma, founded in 2010, is a CRDMO specializing in microbial expression systems. We focus on the development and manufacturing of recombinant proteins, plasmids, nano-bodies, vaccines, mRNA, and circRNA, providing integrated CRO/CDMO/MAH services across the entire biologics lifecycle.
- Expert Team
- Scalable Biomanufacturing
- Rich Project Experience
- Regulatory Compliance

0
+
Years Experience
Overview
Delivering results, reliability, & rock solid dependability.
Integer quam lacinia nibh erat nam. Lobortis nec ullamcorper tincidunt ante feugiat curabitur dui nulla tempus luctus sapien. Sollicitudin ullamcorper iaculis ipsum dui lorem nostra velit parturient semper penatibus.
0
+
Project Done
0
%
Average Success
What we do
Providing One-Stop Microbial CRDMO Solutions
for Biologics
01.
Recombinant
Protein
Protein
01. Recombinant Protein
Comprehensive and integrated CRDMO platform for recombinant proteins and peptide therapeutics
Learn more 02.
Plasmid DNA
02. Plasmid DNA
GMP-compliant one-stop CRDMO platform for plasmid development, commercial manufacturing, and regulatory submission
Learn more 03.
Nano-body
03. Nano-body
Microbial recombinant expression systems providing integrated, end-to-end nano-body CRDMO services
Learn more 04.
Vaccine
04. Vaccine
Focusing on microbial expression systems, with comprehensive development of diverse recombinant vaccines (VLP and DNA vaccines)
Learn more 05.
mRNA
05. mRNA
One-stop mRNA CRO solutions delivering research-grade, custom mRNA synthesis services
Learn more 06.
CircRNA
06. CircRNA
High-quality circRNA custom services covering the full lifecycle of research-grade circRNA sample preparation and quality control
Learn more ISO & EU QP Certified Microbial CRDMO Solutions
Yaohai Bio-Pharma is ISO-certified and EU QP-audited, reflecting our firm commitment to global quality standards and service excellence.

Expert in Microbial CRDMO
Shaping the Future of Biomanufacturing
Yaohai Bio-Pharma is a customer-centric company specializing in diverse biologics manufacturing and process development,
and it provides GMP-compliant, high-quality microbial CRDMO solutions.
and it provides GMP-compliant, high-quality microbial CRDMO solutions.
0
+
Global Clients
0
+
Delivered Projects
0
+m²
GMP Manufacturing Area
0
+
Successful Audits
From R&D to Commercialization
End-to-End Microbial CRDMO Solutions
CMC Process Development Platform
We provide comprehensive CMC process development services, covering microbial cell banking, upstream and downstream process development, and formulation process development.
GMP Manufacturing Platform
Our manufacturing processes comply with GMP standards, ensuring the production of drug substances and drug products for both clinical and commercial applications.
Quality Control Platform
We offer complete analytical support, covering raw material testing, in-process control, release testing, and stability studies to ensure product quality and compliance.
Global Regulatory Affairs Platform
We provide comprehensive support for global IND and BLA submissions, including CMC document preparation in CTD format, regulatory consulting, and technical communication with regulatory authorities.
Quality Assurance Platform
Our QA system complies with ISO, ICH, and GMP standards, covering document control, audits, risk management, and continuous improvement, with strict IP and data privacy protection.
Loka Industrial Maluku
Faucibus phasellus placerat aptent semper leo sagittis felis consectetur malesuada taciti per senectus netus auctor
Let's start talking with us
Our value
Serve With Heart
Build Future Together
Yaohai Bio-Pharma delivers tailored CRDMO solutions for global clients, meeting diverse project requirements with excellence and efficiency.
Proven Project Excellence
Proven track record of over 200 successful projects, spanning preclinical studies and Phase I/II/III clinical trials, including US–China dual submissions and Australian regulatory filings.
Regulatory Compliance Assurance
A robust GMP-aligned quality system with standardized SOPs, fully compliant with global regulatory guidelines across the entire product lifecycle.
Expert Team Support
Powered by industry-leading scientists and cross-functional experts, our team delivers CRDMO projects with speed, precision, and deep technical insight.
Need Help?
World’s premiers engineering company.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Customer Support
Ipsum pede habitant letius efficitur condimentum penatibus potenti tortor morbi
Customer Support
Ipsum pede habitant letius efficitur condimentum penatibus potenti tortor morbi
News & Article
Ipsum pede habitant letius efficitur condimentum penatibus potenti tortor morbi
Related Articles


The Overview of DS Manufacturing Expert
December 25, 2025
No Comments
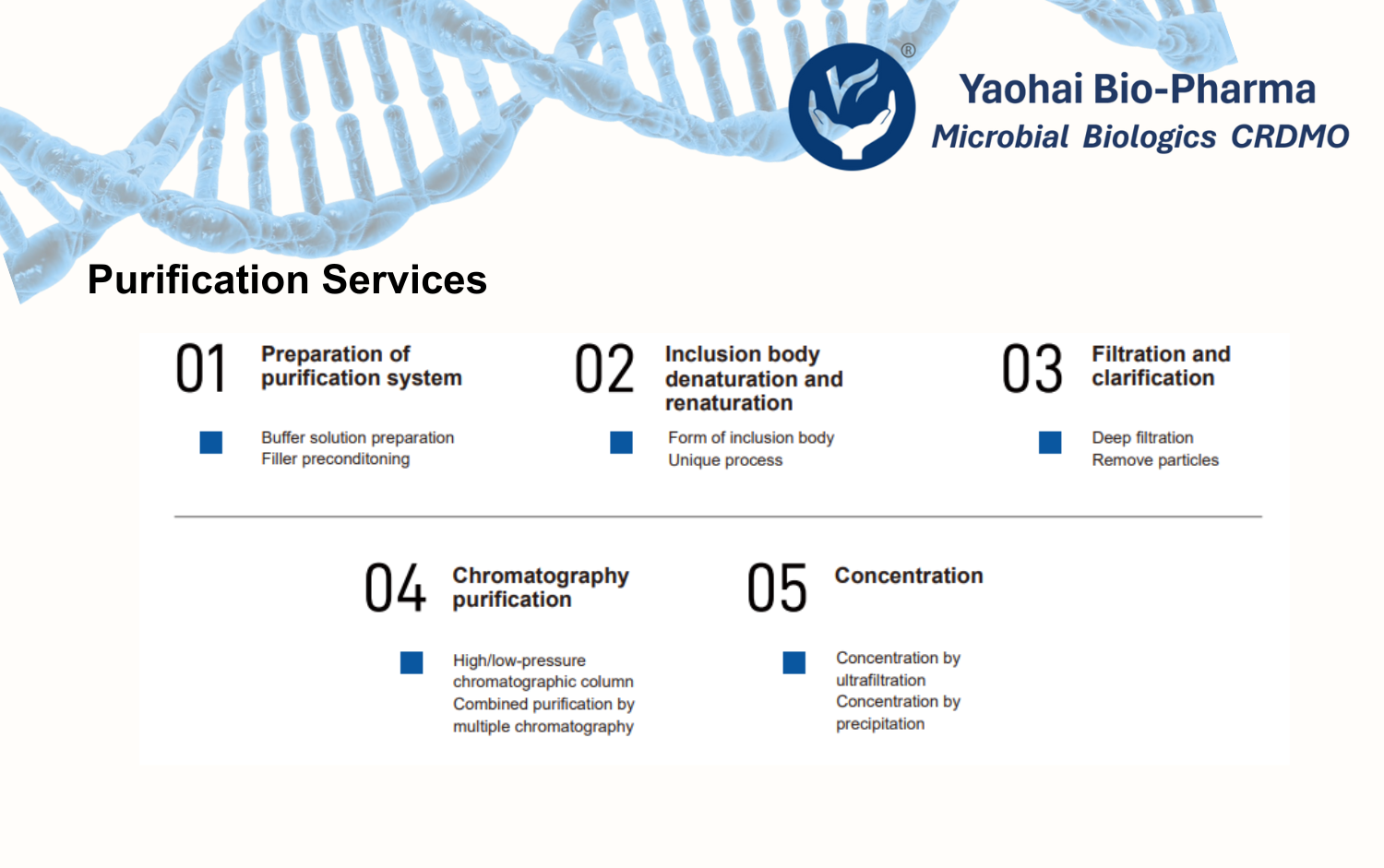
Yaohai Purification is the Best Standard
November 28, 2025
No Comments

