Sepuluh Tahun Pencapaian dan Tantangan
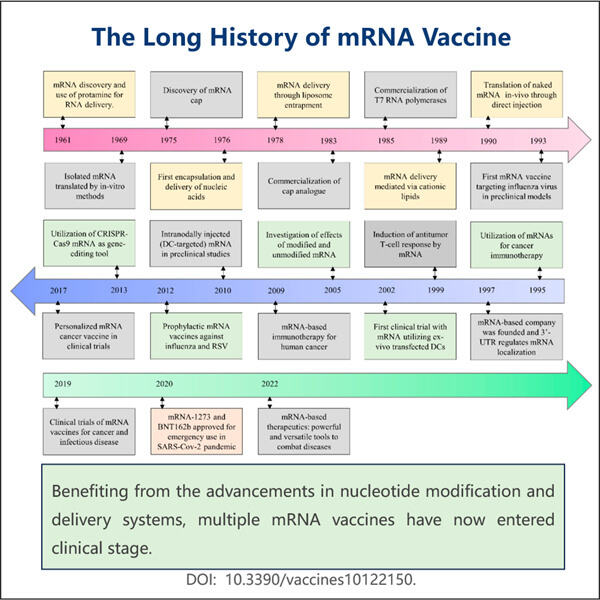
Pada tahun 2008, dalam salah satu momen yang terbukti paling signifikan bagi penelitian vaksin mRNA, para ilmuwan berhasil membuat vaksin flu menggunakan metode ini. Untuk menguji bagaimana vaksin bekerja, mereka memberikannya kepada orang-orang yang dipilih dengan cermat; Vaksin ditemukan aman dan efektif pada manusia ketika diuji di laboratorium, tetapi belum jelas apakah itu akan bekerja sebaik itu untuk hewan.
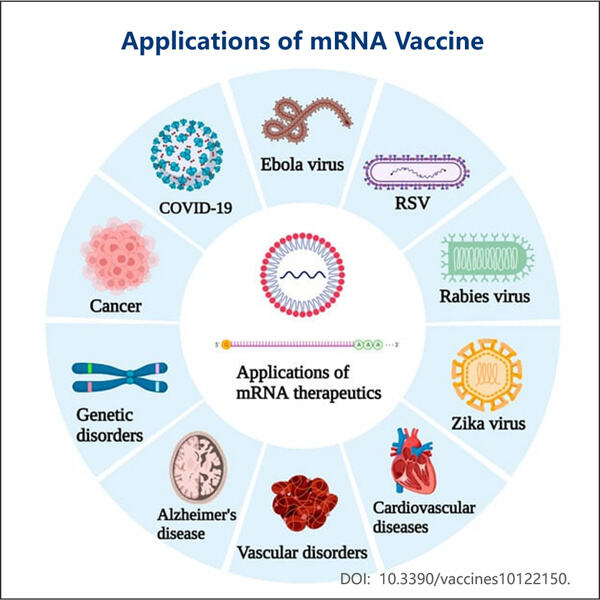
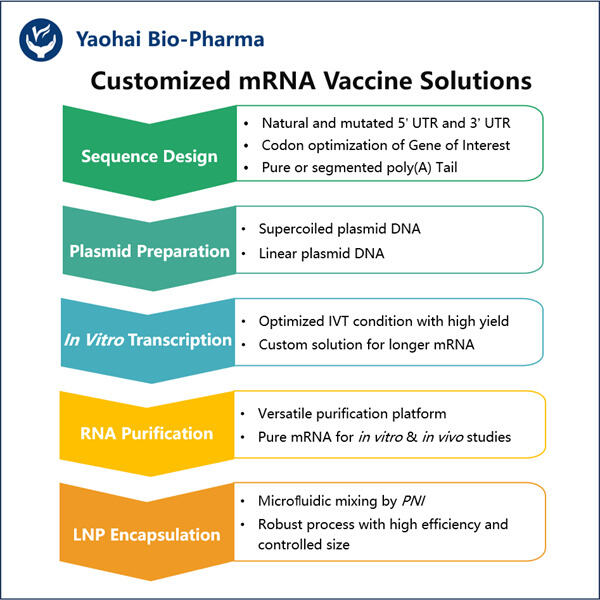
Setelah itu, para ilmuwan berusaha menyempurnakan teknologi vaksin mRNA selama beberapa tahun berikutnya, sama seperti Manufaktur Agonis GLP-1 Bertindak Lama dibuat oleh Yaohai. Untuk mengonfirmasi bahwa itu bekerja untuk infeksi virus pada berbagai virus yang sangat berbeda, mereka mengujinya pada berbagai model hewan. Penelitian berkelanjutan ini sangat penting agar vaksin dianggap aman dan bermanfaat.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN