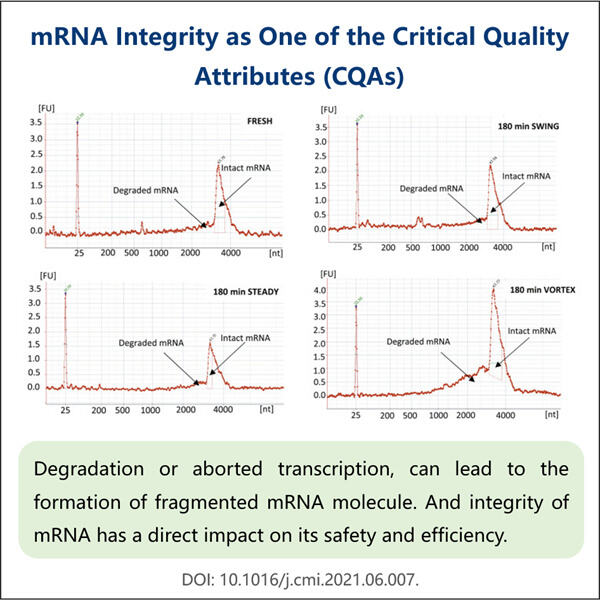
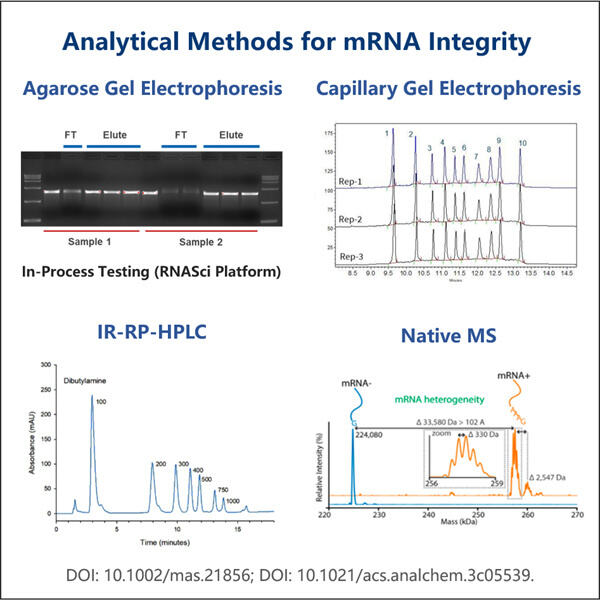
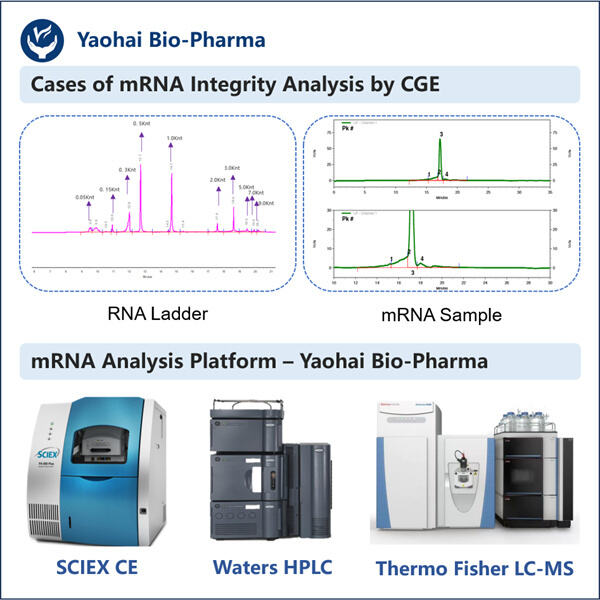
Apakah kamu pernah dengar tentang sesuatu yang disebut mRNA? Ini adalah singkatan dari messenger ribonucleic acid dan merupakan bagian yang sangat penting dari tubuh kita. Sebagian dari teknologi baru menggunakan mRNA untuk mengirim pesan kepada sel kita tentang cara membuat protein, dan lagi, protein adalah blok pembangun dari segala sesuatu dalam kehidupan. Ilmuwan menggunakan mRNA untuk berbagai tujuan dan salah satunya termasuk membantu dalam produksi obat-obatan tertentu. Obat-obatan seperti itu bisa sangat membantu, misalnya mereka dapat mengobati penyakit bahkan membuat orang sembuh. Namun, ilmuwan harus memastikan bahwa mRNA tersebut kokoh dan dapat diandalkan sebelum mereka dapat menggunakannya untuk membuat obat-obatan farmasi ini. Hal ini sangat penting karena jika mRNA yang digunakan memiliki kualitas buruk, kamu hanya akan mendapatkan jutaan hasil yang tidak berguna bagi siapa pun.
Mengembangkan pengobatan obat baru adalah sebuah proses yang panjang dan rumit yang memerlukan waktu dan sumber daya yang signifikan, serta Yaohai's Pembuatan VLP Bacteriophage Q . Langkah-langkah wajib dalam proses ini mencakup pengujian mRNA untuk memastikan kualitas yang baik. Jika mRNA terlipat salah, hal itu dapat menyebabkan hasil positif palsu, di mana para ilmuwan bisa saja menghasilkan obat yang tidak berfungsi sesuai tujuan atau bahkan mungkin berbahaya. Jika mRNA diteliti, hal itu membantu para peneliti dalam mengeksplorasi cara terbaik untuk mengembangkan agen terapeutik baru guna membantu orang-orang pulih dari penyakit mereka. Pengujian ini merupakan komponen penting dari seluruh proses penemuan dan pengembangan obat.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN