ما هو إنتاج RNA الطويل بواسطة IVT؟ يبدو الأمر غريبًا بعض الشيء ولكنه في الواقع واحد من أهم الأمور في العلم، خاصة عندما يتعلق الأمر بالطب. ستتعلم كل شيء عن إنتاج RNA الطويل بواسطة IVT، ما الذي يفعله، لماذا هو مهم جدًا، وكيف يؤثر على نجاح شيئ مثل العلاج الجيني أو اللقاحات. ستجد أيضًا شركة تُدعى ياوهاي مكرسة لتحسين وإتقان إنتاج RNA الطويل بواسطة IVT.
ولكن إذا كان علينا أن نفهم إنتاج RNA الطويل عن طريق IVT وكيف يتم تطبيقه في البحث/الطب، فلنبدأ أولاً بمعرفة المعنى الدقيق للعبارة. RNA الطويل عن طريق IVT. RNA التفاعلية خارج الجسم (In Vitro Transcription). كل هذا يبدو معقدًا، لكن في جوهره يعني أن العلماء قد أنتجوا RNA خارج الكائنات الحية. عبارة 'In vitro' تعني أن العملية تحدث داخل المختبر وليس داخل جسم حي.
حل إنتاج RNA الطويل عن طريق IVT هو أداة مفيدة، لكن عملية إنتاج RNA الطويل عن طريق IVT لديها بعض القيود. أحد المشاكل الرئيسية هو كيفية تحسين جميع العمليات في الإنتاج. كفاءة: لإنجاز العمل بسرعة وبجهد قليل. قامت Yaohai بإنتاج RNA الطويل عن طريق IVT تصنيع بلازميد mRNA لمدة تزيد عن 10 سنوات. إنهم يصنعون أدوات جديدة تُسمى الإنزيمات التي يمكن أن تساعدهم على تسريع العملية وتحسينها أيضًا.
المشكلة الثانية التي تواجه العلماء هي ضمان منتجهم من CircRNA الإنتيرلوكين RNA هو باللون المناسب ولديه التسلسل الصحيح. يجب أن تكون RNA دقيقة، لأن هذا يحدد مدى كفاءتها أثناء التجارب والعلاجات. بالإضافة إلى ذلك، تعمل خدمة إنتاج RNA الطويلة IVT أيضًا على تحقيق ذلك. بهذه الطريقة، يمكن للباحثين أن يكون لديهم ضمان أفضل بأن RNA الذي يستخدمونه هو بالفعل ذو جودة عالية ومجهز للعمل كما هو متوقع.
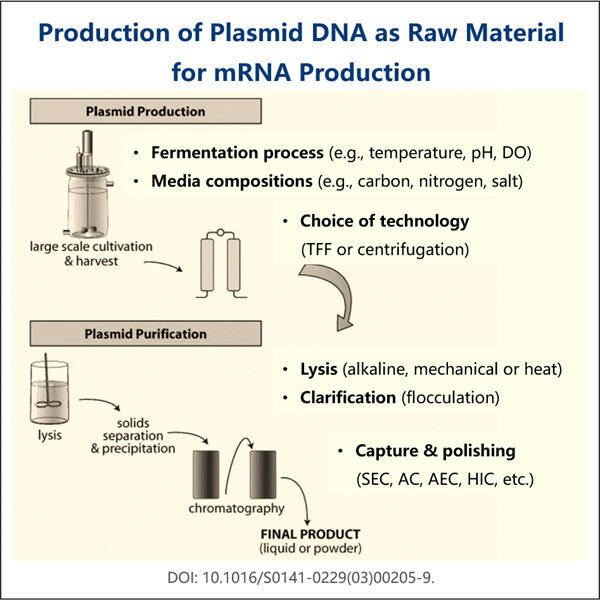
كما نشير أعلاه، أحد أكبر المعضلات التي تحاول حلها تصنيع لقاح VLP-RNA هو كيفية تحسين هذه العملية بشكل كبير. يعمل Yaohai الرائد على القيام بنفس الشيء القديم دون الإرهاق، لكن مجموعة جديدة من الإنزيمات لا تزال تقوم بذلك بكفاءة أكبر. وهذا يعني، بين أمور أخرى، أن العلماء يمكنهم صنع RNA بسرعة أكبر، وأهم من ذلك، بدء البحث ومعالجة المرض في وقت أقرب.
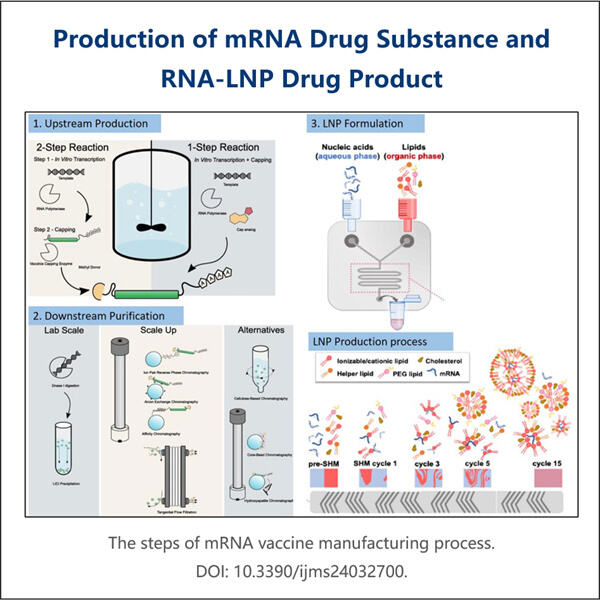
ولكن واحدة من أكبر التطبيقات العملية لتصنيع RNA طويل IVT هي في بناء وتصنيع اللقاحات القائمة على RNA. الفكرة وراء هذه اللقاحات، بشكل أساسي، هي أنها تعمل عن طريق تقديم تعليمات دقيقة للجسم عبر RNA حول كيفية استجابته لتهديد معين. RNA هو الرسول الأساسي في خلايانا، حيث ينقل المعلومات الوراثية التي تخبر الجهاز المناعي بالتعرف على وتحييد مثل هذه التهديدات. قدومRNA الاصطناعي إلى الجسم من خلال التطعيم يجعل الجهاز المناعي أكثر قدرة على التعرف والرد بفعالية ضد مسببات محددة للأمراض. بعد ذلك، يُتاح له فرصة بناء دفاعات مركزية مثل الأجسام المضادة قبل التعرض للمرض الحقيقي. وهذا يعني أن اللقاحات القائمة على RNA تكون مفيدة للغاية في الوقاية وتقليل تأثير الأمراض المعدية، مما يجعل الجسم أكثر قدرة على منع الأمراض المستقبلية.
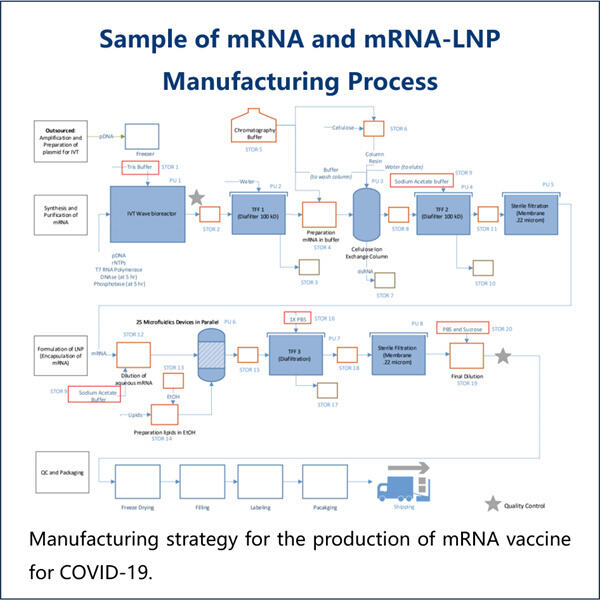
إحدى المجالات التي تثير الاهتمام بشكل خاص في ياوهاي هي استخدام RNA الطويل المُنتج بواسطة IVT لعلاج السرطان. السرطان هو مرض خطير للغاية، والأمر الأكثر أهمية هو اكتشاف العلاجات. يمكن أن يكون علاج RNA الطويل المُنتج بواسطة IVT قادراً على استهداف الخلايا السرطانية من خلال إدخال جزيئات RNA موجهة لعلاج الأورام الصعبة بشكل خاص. يمكن لهذا أن يقدم أملًا لعدد لا يحصى من الناس الذين يقاتلون السرطان ويعطيهم فرصة للقتال.
ياوهاي بايو-فارما لديها خبرة في تطوير الأدوية البيولوجية المستخلصة من الميكروبات. نقدم حلول RD مخصصة والتصنيع مع الحفاظ على المخاطر عند أدنى مستوى. لقد استخدمنا مجموعة متنوعة من الطرق بما في ذلك الوحدات المركبة рекombinant (تشمل الببتيدات)، عوامل النمو، الهرمونات، والسايتوكنات. تخصصنا يشمل عدة كائنات دقيقة مثل الخميرة السكرية، الإفراز الخلوي الداخلي والخارجي (الإنتاج يصل إلى 15 جم/لتر) والبكتيريا، الذوبان الخلوي الداخلي وجسم التضمين (الإنتاج يصل إلى 10 جم/لتر). كما قمنا بإنشاء نظام تخمير لإنتاج RNA IVT طويل لتطوير اللقاحات القائمة على البكتيريا. نحن خبراء في تحسين العمليات وزيادة الإنتاج وتقليل تكاليف الإنتاج. باستخدام فريق تقني قوي، نضمن تسليم المشاريع في الوقت المناسب وبجودة عالية لجلب منتجاتك الحصرية إلى السوق بشكل أسرع.
ياوهاي بايو فارما، واحدة من أكبر 10 شركات CDMO المجهرية التي تدمج إدارة الجودة والشؤون التنظيمية. نظام جودتنا متوافق مع معايير GMP الحالية واللوائح الدولية. فريق خبراء الشؤون التنظيمية لدينا متمرس في الإطارات التنظيمية العالمية لتسريع إطلاق المنتجات البيولوجية. نحن نضمن إجراءات إنتاج قابلة للتعقب، ومنتجات ذات جودة عالية، بالإضافة إلى الامتثال لمتطلبات إنتاج RNA الطويل IVT وEMA الأوروبية. كما يتم الالتزام بالمتطلبات الخاصة بـ TGA الأسترالية وNMPA الصينية. لقد نجحت ياوهاي بايو فارما في اجتياز التدقيق الشخصي الذي أجرته شخصية مؤهلة من الاتحاد الأوروبي (QP) لفحص نظام GMP ومرافق الإنتاج لدينا. كما أكملنا عمليات التدقيق الأولية لنظام إدارة الجودة ISO9001 ونظام إدارة البيئة ISO14001.
ياوهاي بيولوجي فارما هي رائدة في مجال CDMO للميكروبات الحيوية. تركيزنا الأساسي هو إنتاج اللقاحات والعلاجات الميكروبية لإدارة الحيوانات الأليفة، الإنسان وإنتاج RNA الطويل IVT. نحن مجهزون بمنصات RD المتقدمة والتكنولوجيات التصنيعية التي تغطي العملية بأكملها بدءًا من تطوير سلالات الميكروبات وبنوك الخلايا، إلى تطوير الطرق والعمليات، وحتى التصنيع السريري والتجاري الذي يضمن توفير حلول جديدة بنجاح. عبر السنوات، تراكم لدينا معرفة واسعة حول معالجة البيولوجيا القائمة على الميكروبات. تم إكمال أكثر من 200 مشروع بنجاح ونساعد عملائنا على تجاوز اللوائح مثل تلك الخاصة بالهيئة الأمريكية للدواء والغذاء (FDA) والهيئة الأوروبية للأدوية (EMA). كما نساعد العملاء في التعامل مع TGA الأسترالية وNMPA الصينية. نحن قادرون على الاستجابة بسرعة لاحتياجات السوق وتقديم خدمات CDMO مخصصة بفضل خبرتنا ومهارتنا.
ياوهاي بيو-فارما، واحدة من أكبر 10 شركات لإنتاج RNA الطويل باستخدام IVT، وهي متخصصة في التخمير المجهرى. لقد أنشأنا منشأة حديثة تمتلك قدرات بحث وتطوير قوية وبنية تحتية متقدمة. هناك خمس خطوط إنتاج للأدوية المطابقة للمعايير GMP لتنقية وتخمير الخلايا الدقيقة، بالإضافة إلى خطين لتعبئة الأنبوب وزجاجات الحقن المسبقة التعبئة. تتراوح أحجام التخمير المتاحة بين 100 لتر و2000 لتر. مواصفات التعبئة للأنابيب تتراوح بين 1 مل و25 مل، بينما تكون متطلبات التعبئة للحقن أو الكروتridge المسبقة التعبئة بين 1-3 مل. ورشة الإنتاج معتمدة حسب cGMP وتقدم عينات سريرية وتجارية. يمكن تسليم الجزيئات الكبيرة التي يتم تصنيعها في منشأتنا إلى جميع أنحاء العالم.