الأدوية الحيوية هي مواد ينشئها العلماء عندما يطورون دواءً لمساعدة المرضى. نظرة أقرب على الأدوية الحيوية تظهر أنها أنواع فريدة من الأدوية التي يمكنها علاج الأمراض من خلال استهداف مناطق محددة من الجسم البشري. ومع ذلك، فإن إنتاج هذه الأدوية هو عملية صعبة ومملة تستهلك الكثير من الموارد. ياوهاي إنتاج VHH ثنائي المواصفات : نوع من الأدوية الحيوية الذي يؤدي بالكثير من الناس إلى العين هو VHH ثنائي القيمة.
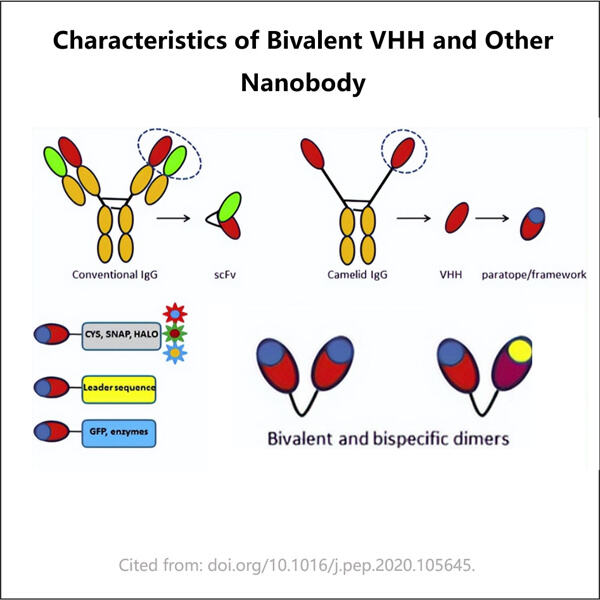
كما هو مذكور أعلاه، تعتبر جزيئات VHH ثنائية القيمة خاصة للغاية لأنها صغيرة جدًا ولذلك لديها خاصية الانتشار في جميع أنحاء أجسام الإنسان. وهذا يمنحها الوصول إلى الأعضاء المصابة. بالإضافة إلى ذلك، فإن جزيئات VHH ثنائية القيمة أصغر بكثير من الأجسام المضادة العادية التي تُستخدم عادةً لتكوين الأدوية البيولوجية، بينما كانت خلايا مبيض الجربوع الياباني معروفة كخط خلايا ثديية لإنتاج كبير من الجسم المضاد IgG في الواقع. هذا الحجم الصغير هو ما يجعل VHH ثنائي القيمة منصة لقاح ممتازة لتطوير العلاجات التي يمكن أن تنقذ حياة المرضى وتجعلهم يشعرون بالتحسن.
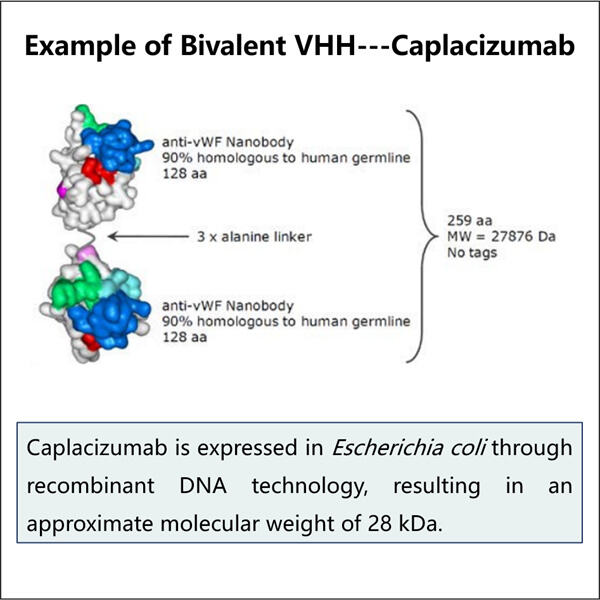
من أجل صنع هذه الأنواع من الجزيئات، يتطلب ذلك برامج كمبيوتر خاصة تساعدنا في تصميمها ياوهاي إنتاج VHH مضاد لـ vWF حتى يتمكنوا من الالتصاق بالبروتينات المحددة الموجودة في الإنسان. ثم نطبق عدة عمليات متقدمة لإنتاج الكثير من هذه الجزيئات دفعة واحدة - كل ذلك في أمل أن نتمكن من إنتاج كمية كافية من الدواء لعدد كبير جدًا من المرضى.
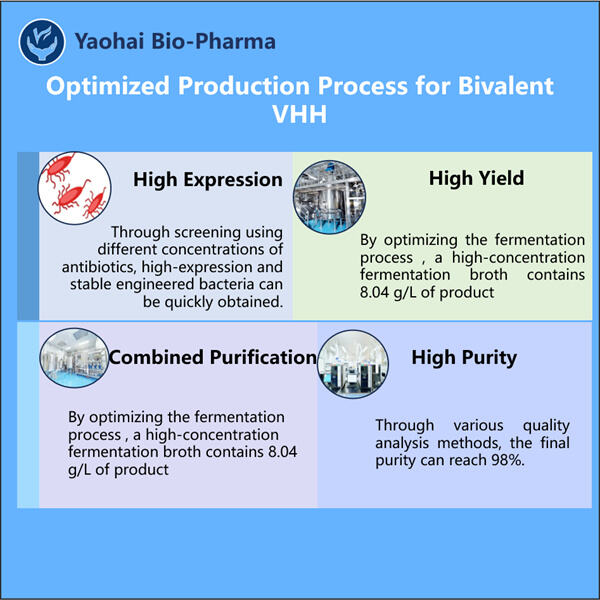
إنتاج كمية كافية من المنتجات البيولوجية لتلبية الطلب على الأشخاص الذين يحتاجون إليها هو أحد أكثر القضايا إزعاجًا في إنتاجها. وهذا يعود جزئيًا إلى حقيقة أن المنتجات البيولوجية هي جزيئات بروتينية حساسة قد تتدهور بسهولة إذا لم يتم تخزينها ونقلها بشكل مناسب. ساعدتنا استراتيجياتنا الابتكارية في إنتاج كمية ضخمة من جزيئات VHH ثنائية القيمة بطريقة أذكى لا تؤثر إطلاقًا على جودتها أو فعاليتها.
في ياوهاي، ما نريد القيام به هو صنع علاجات جيدة للمرضى الحقيقيين. لذلك قمنا بإنشاء طريقة إنتاج ممتازة للـ VHH ثنائي القيمة لاستخدامها في العلاج. تم تخصيص جزيئات VHH ثنائية القيمة لاكتشاف البروتينات المسؤولة عن التسبب في حالات مرضية مختلفة في النظام البشري. من خلال استهداف هذه البروتينات، يمكن لجزيئات VHH ثنائية القيمة تخفيف الأعراض المرتبطة بهذه الأمراض وتحقيق تحسن مطلوب في حياة العديد من المرضى هناك.
إحدى مزايا جزيئات VHH ثنائية القيمة هي أن هذه يمكن برمجتها لتحقيق خصوصية معينة للبروتينات داخل الجسم. وبالتالي، يمكننا تصميم الدواء لكل مريض على حدة ومصمم خصيصًا لمساعدته على التعامل مع مشاكله الصحية. في ياوهاي، قمنا بتصميم نهج جديد لإنتاج VHH ثنائي القيمة الذي يمكن استخدامه لعلاج العديد من الأمراض.
مع تطوير إنتاجنا للـ VHH ثنائي القيمة، على وشك أن تتغير الأدوية الحيوية للأفضل في ياوهاي. طريقة جديدة من ياوهاي إنتاج VHH ثلاثي القيمة وكذلك القدرة على إنتاج وتصنيعها لأغراض علاجية، والتي يتم تكييفها مع الطب المخصص الذي يُصنع لتحسين جودة حياة المرضى ومساعدة العديد من المرضى.
تتمتع Yaohai Bio-Pharma بخبرة واسعة في تطوير الأدوية البيولوجية المستخلصة من الميكروبات. نقدم حلولًا مخصصة للبحث والتطوير وكذلك التصنيع، مع التأكد من عدم وجود أي مخاطر. عملنا على أنماط متنوعة مثل اللقاحات рекombinانت القائمة على الوحدات الفرعية، تصنيع VHH ثنائي القيمة، السيتوكينات، عوامل النمو، الأجسام المضادة ذات المجال الواحد، الإنزيمات، الحمض النووي البلازميدي، الرنا المرسال، وغيرها. نحن خبراء في مجموعة متنوعة من الكائنات الدقيقة، بما في ذلك إفراز الخلايا الخارجية والداخلية لخميرة (مع إنتاج يصل إلى 15 جم/لتر) وكذلك الذوبان داخل الخلية وإنتاج الجسم الشامل للبكتيريا (مع إنتاج يصل إلى 10 جم/لتر). كما طورنا منصة التخمير BSL-2 لإنشاء لقاحات قاعدية على البكتيريا. لدينا سجل حافل في تحسين العمليات الإنتاجية، مما يؤدي إلى زيادة الإنتاج وتقليل التكاليف. ومع فريق تقني كفؤ للغاية، نضمن تسليم المشاريع في الوقت المحدد وبجودة عالية، مما يساعد على إدخال منتجاتكم إلى السوق بشكل أسرع.
تصنيع VHH ثنائي القيمة هو أحد أفضل 10 شركات CDMO الدقيقة التي تدمج التحكم في الجودة والقضايا التنظيمية. لقد أنشأنا نظام جودة قوي يتوافق مع معايير GMP الحالية واللوائح في جميع أنحاء العالم. فريقنا التنظيمي على دراية واسعة بالإطارات التنظيمية العالمية لتسريع إطلاق المنتجات البيولوجية. نضمن عمليات إنتاج قابلة للتتبع ومنتجات ذات جودة عالية تلتزم بقواعد US FDA، EU EMA، Australia TGA، و China NMPA. تمكنت Yaohai BioPharma من النجاح في الفحص الميداني الذي أجرته شخصية مؤهلة معتمدة من الاتحاد الأوروبي (QP) لمراجعة نظام GMP الخاص بنا ومرافق الإنتاج. بالإضافة إلى ذلك، قد مررنا بنجاح أول تدقيق للشهادات لنظام إدارة الجودة ISO9001، نظام إدارة البيئة ISO14001، ونظام إدارة الصحة والسلامة المهنية ISO45001.
ياوهاي بايو-فارما هي رائدة في مجال خدمات CDMO الحيوية الدقيقة. يركز عملنا بشكل أساسي على إنتاج اللقاحات والعلاجات الحيوية الدقيقة لإدارة صحة الحيوانات الأليفة، البشر، وإنتاج VHH ثنائي القيمة. نحن مجهزون بتقنيات بحث وتطوير متقدمة وتقنيات تصنيع تغطي العملية بأكملها، بدءًا من تطوير سلالات دقيقة وبنوك الخلايا، إلى تطوير الأساليب والعمليات، وحتى التصنيع السريري والتجاري لضمان توفير حلول جديدة بنجاح. عبر السنوات، اكتسبنا خبرة واسعة في معالجة البيولوجيات القائمة على الكائنات الدقيقة. تم تنفيذ أكثر من 200 مشروع بنجاح، ونساعد عملاءنا على تجاوز اللوائح مثل تلك الخاصة بالهيئة الأمريكية للدواء والغذاء (FDA) والهيئة الأوروبية للأدوية (EMA). كما نساعد العملاء في التعامل مع الهيئة الأسترالية TGA وهيئة NMPA الصينية. نستطيع الاستجابة بسرعة لمتطلبات السوق وتقديم خدمات CDMO مخصصة بفضل خبرتنا ومهارتنا.
ياوهاي بايو-فارما، واحدة من أكبر 10 مصنعي المنتجات البيولوجية، تخصصت في التخمير المجاري. لقد أنشأنا منشأة تصنيع VHH ثنائية القيمة بقدرات قوية في البحث والتطوير ومرافق تصنيع متقدمة. هناك خمس خطوط إنتاج مواد دوائية تتوافق مع معايير GMP لتنقية وتخمير الخلايا المجهرية، بالإضافة إلى خطين لتعبئة الأدوية في الزجاجات والكروتيدات وكذلك الإبر المسبقة التعبئة. تتاح أحجام التخمير بما في ذلك 100لتر، 500لتر، 1000لتر و2000لتر. مواصفات تعبئة الزجاجات تتراوح بين 1مل - 25مل. أما مواصفات تعبئة الكروتيدات أو السرنجات المسبقة التعبئة فتتراوح بين 1-3مل. ورشة الإنتاج متوافقة مع cGMP وتقدم إمداداً مستقراً بالمنتجات التجارية والعينات السريرية. منشأتنا تنتج الجزيئات الكبيرة التي يتم شحنها عالميًا.