Ilmu di Balik Antibodi Monoklonal VHH
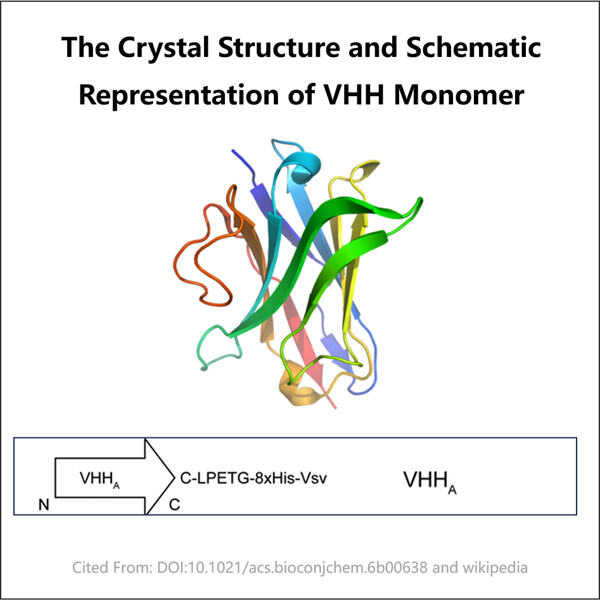
Rahasia di balik Antibodi Monoklonal VHH: Satu Protein Organisme. Hal ini membuatnya lebih kompak dan fleksibel daripada antibodi yang dibuat oleh tubuh kita untuk melawan infeksi. Berbeda dengan Antibodi Normal, ketika mengikat targetnya, antibodi normal memberi tahu sistem kekebalan tubuh inang yang kemudian mengenali dan menghancurkannya. Selain itu, karena kemampuan mereka untuk bergerak di dalam tubuh sangat baik, mereka menjadi nanopartikel yang sangat baik untuk deteksi dan pengobatan penyakit.
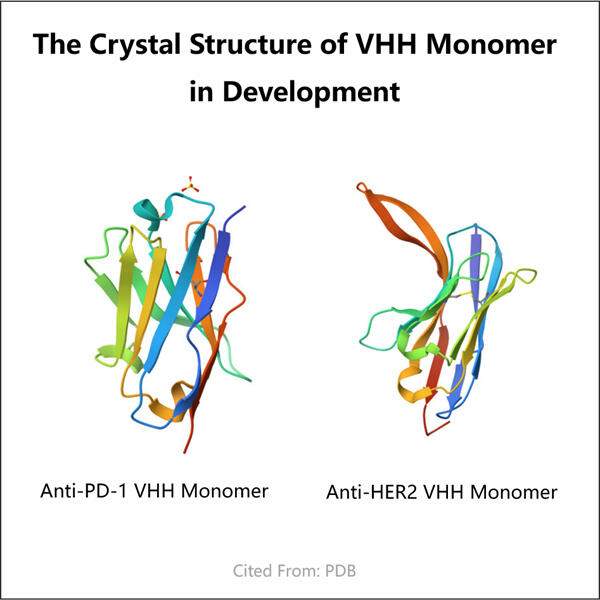
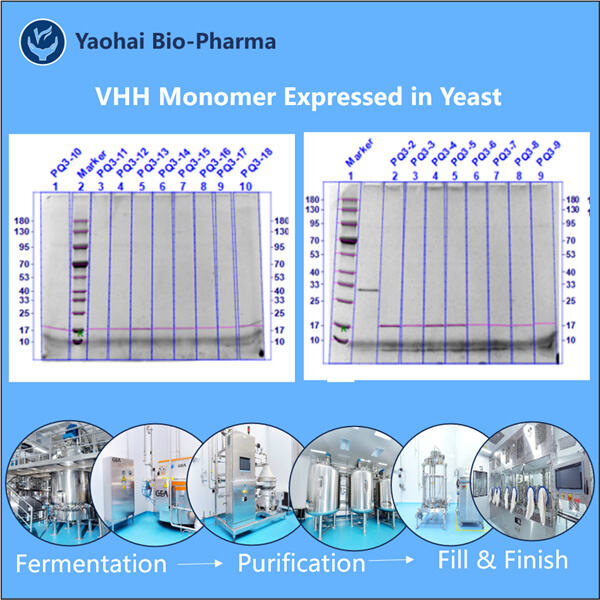
Membuat Antibodi Monoklonal VHH bukanlah tugas yang sederhana. Dibutuhkan teknologi Hi-Fi dan peralatan khusus serta keahlian ilmuwan manusia. Namun, masalah dengan pendekatan terakhir adalah bakteremia yang baik untuk sekresi protein. Lagi pula, itu hanya kelemahan jika bakteri tidak berada dalam kondisi stabil memproduksi protein yang cukup untuk Yaohai Pembuatan VHH Bivalen . Tentu saja mereka mendapatkan protein untuk bekerja lebih baik, mereka hanya perlu mengubah protein tersebut. Para ilmuwan, bagaimanapun, harus memanipulasi protein itu sendiri agar dapat ditemukan dan berikatan satu sama lain secara berturut-turut. Namun, protein tersebut perlu stabil untuk sejumlah besar aplikasi ilmiah dan medis.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN