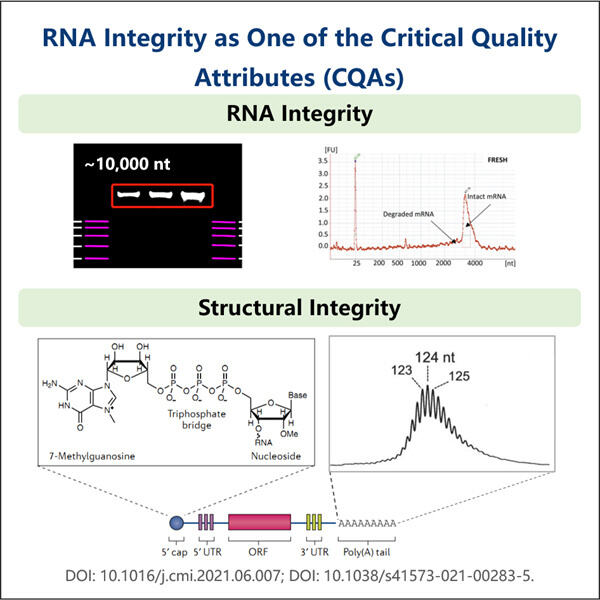
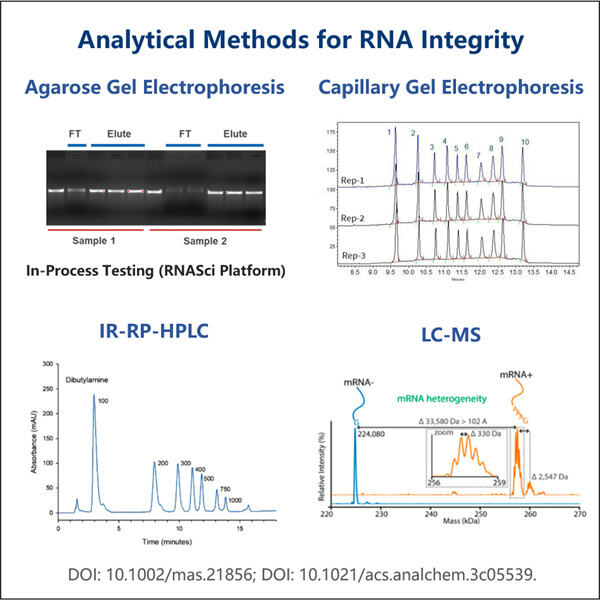
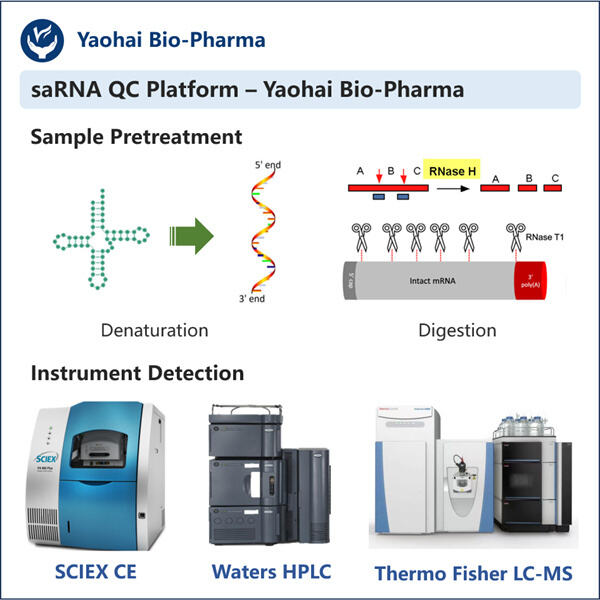
Sicherstellung zuverlässiger Ergebnisse für Gentranskription
Für eine wirksame Gentrapiemethode muss jedoch die test der Kappeffizienz von SaRNA vollständig und optimal funktionieren. Forscher können daher SaRNA vor dem experimentellen Gebrauch testen und sich selbst sicher sein, dass sie mit qualitativ hochwertigen Materialien arbeiten, die effektiv funktionieren. Diese Validierung ist kritisch, da sie den Forschern viel verschwendete Zeit und Ressourcen auf SaRNA erspart, das möglicherweise nicht so funktioniert, wie es erwartet wird.
Stell dir vor, das Testen der SaRNA-Integrität wäre der (wichtige) Schritt, zu überprüfen, ob deine Rezeptszutaten in gutem Zustand sind. Dein Gericht kann nicht abgeschlossen werden, wenn du eine wesentliche Zutat fehlt oder sie verfault oder über dem Ablaufdatum ist. Das Testen des komplementären Aspekts – SaRNA-Integritätstests – hilft Wissenschaftlern sicherzustellen, dass sie alle ihre Dinge geordnet haben und nichts falsch oder kaputt ist.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN