Was ist saRNA (saRNA) und wie funktioniert es? Dies ist eine ungewöhnliche Art von RNA, selbstverstärkende RNA - saRNA, die sich selbst replizieren kann. Hier treffen zelluläre Prozesse zusammen: RNA, eines der kleinen großen Monster in unserem Körper. Diese Proteine übernehmen viele wesentliche Funktionen in unserem Körper, wie die Entwicklung von Muskeln, das Bekämpfen von Infektionen und das Wachstum des Körpers. Die Zukunft ist hell mit verschiedenen Anwendungen dieser saRNA-Technologie, möglicherweise stärker für Impfstoffe und Krankheitsbehandlungen, es wird sehr interessant bis ansprechend für Wissenschaftler aufgrund der hohen Effizienz im Vergleich (14).
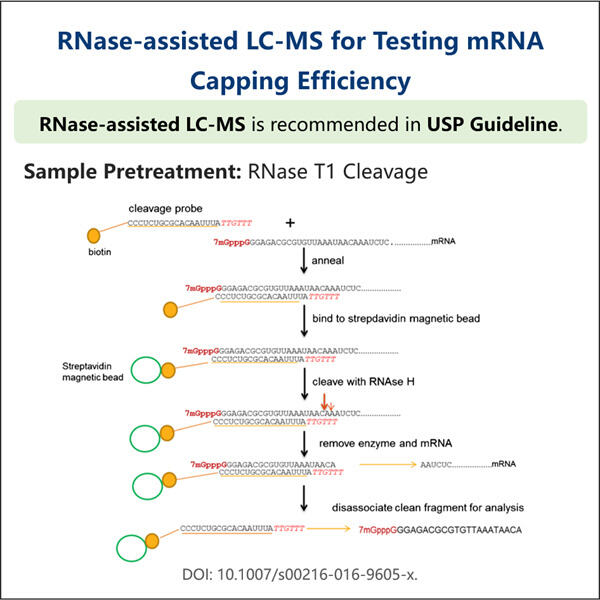
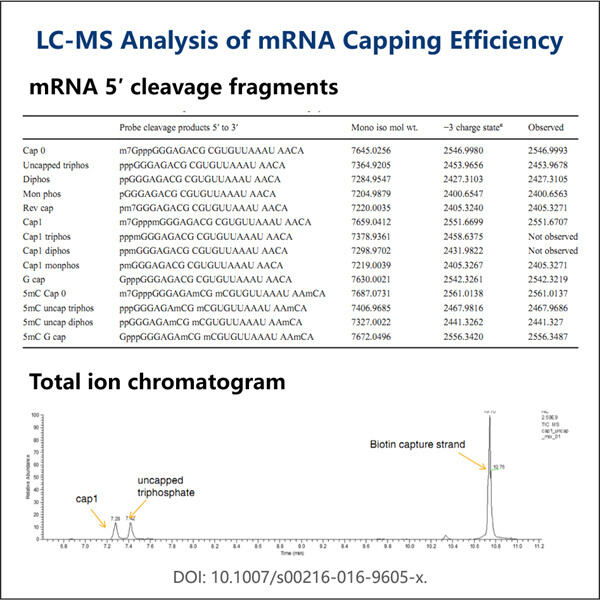
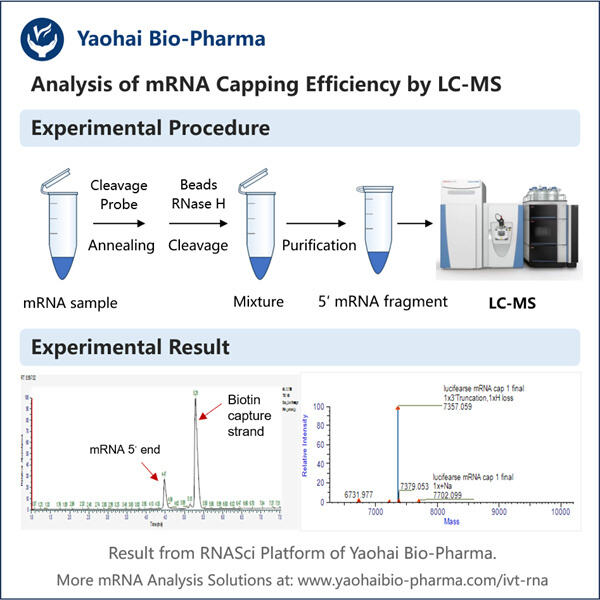
Nun sprechen wir über Capping. Capping ist die Hinzufügung einer eindeutigen Kappe am 5′-Ende von neu entstehenden RNA-Transkripten. Dieser Teil dient als Schutzbarriere, um die RNA vor Zerfall in unseren Zellen zu bewahren. Es hilft der Zelle auch dabei, die RNA zu erkennen, damit sie sie richtig zur Proteinproduktion nutzen kann, die unser Körper benötigt. Dieses Capping ist für saRNA-Therapien notwendig, ansonsten kann die RNA nicht genutzt werden oder könnte sogar zerstört werden.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN