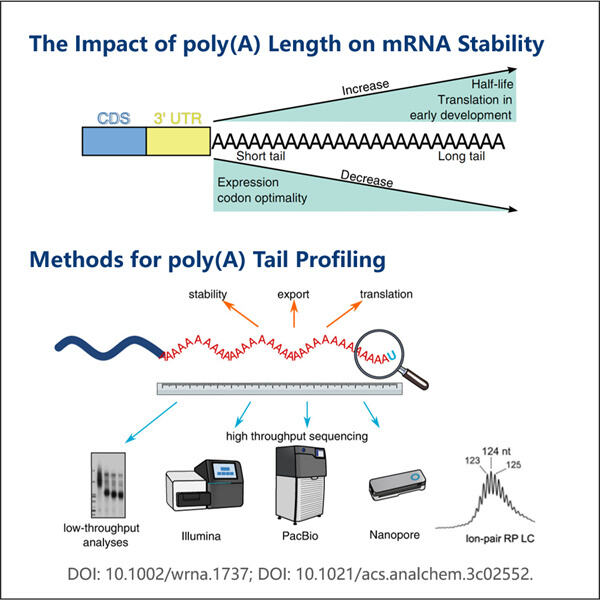
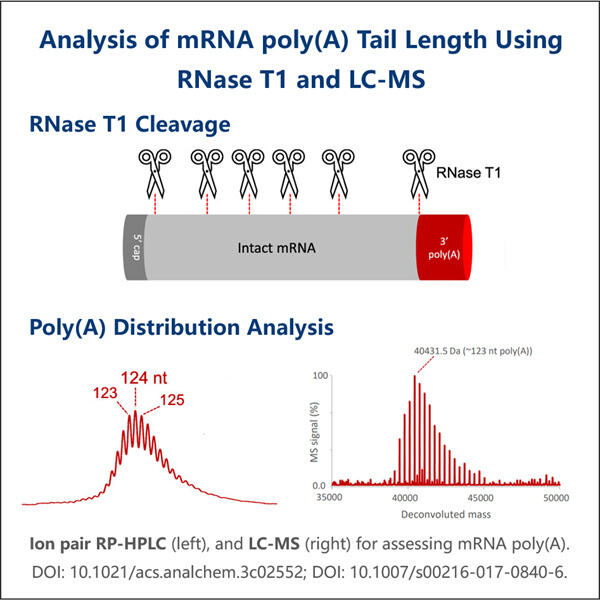
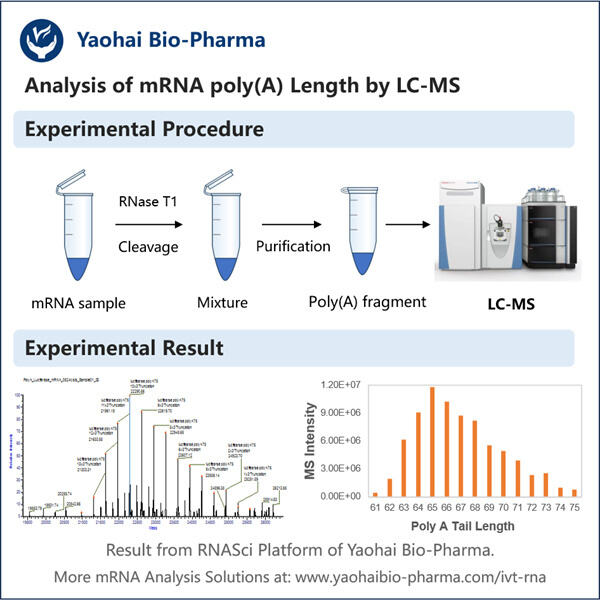
Es gibt eine Million Dinge in der Wissenschaft zu studieren, damit wir verstehen können, wie lebende Wesen funktionieren. Eines dieser wichtigen Themen ist etwas, das mRNA Poly-A genannt wird. Während sich dieser Begriff vielleicht ein bisschen wie Unsinn anhört – ich bin hier, um es in einfachen Worten zu erklären. Das Wissen über mRNA Poly-A ist entscheidend, da es Aufschluss darüber geben kann, wie Gene funktionieren – sie sind die Anweisungshandbücher, die unser Verständnis des Körpers erklären. Das Unternehmen Yaohai ist sehr gut in derartigen Tests. Sie sind technisch versiert und erfahren im Studium von mRNA Poly-A. Warum graben wir nicht tiefer ein und lernen heute mehr darüber?
Was ist es? mRNA steht für Messenger-Ribonukleinsäure. Eine sehr spezielle Molekül, das wertvolle Informationen von unserer DNA zu unseren Zellen transportiert, ähnlich wie Yaohais Produkt wie GMP-Fertigung rekombinanter Peptide . Zum Beispiel betrachten wir die Verwendung von mRNA als Botin, die Anweisungen zur Bildung von Proteinen, Teilen unseres Körpers, transportiert. Im Falle des Poly-A bedeutet dies, dass es einen ungewöhnlichen Schwanz gibt, der am Ende der mRNA hängt. Der Schwanz besteht aus einer Million kleiner Stücke, jedes davon nennt man Adenin. Dann wird dieser Schwanz äußerst wichtig, da er die mRNA gesund und intakt hält. Er ist auch ein Schlüsselteil dafür, wie Gene funktionieren und wie lange sie in unserem Körper aktiv sind.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN