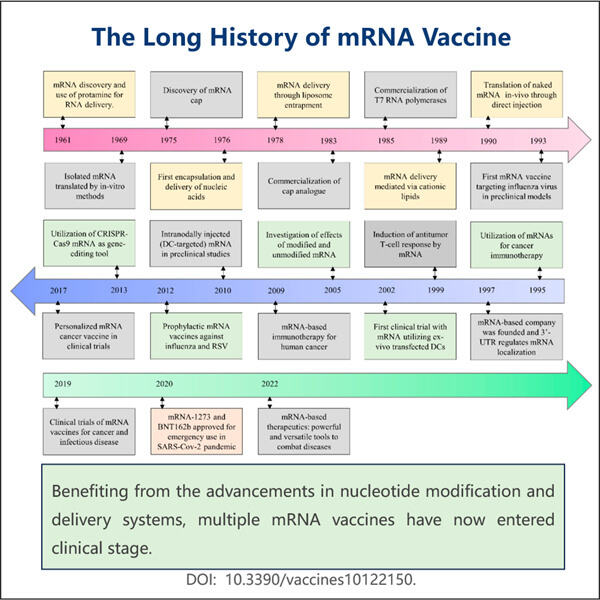
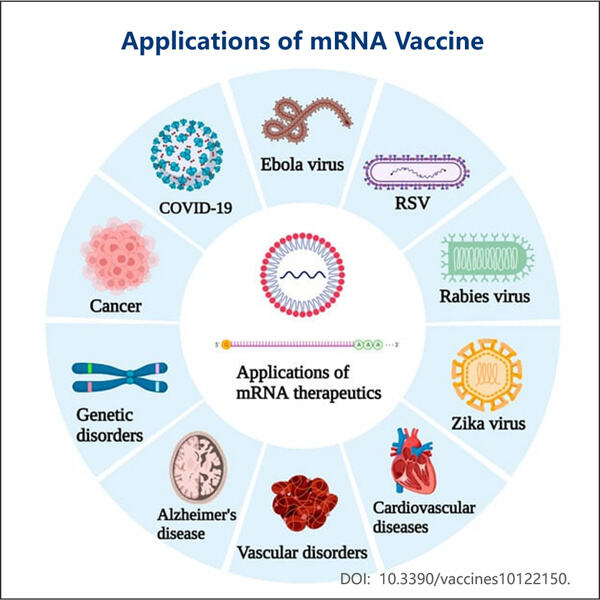
Die Geschichte der mRNA-Impfstoffe im Rückblick (1960er Jahre) Damals entdeckten Wissenschaftler es: Etwas, das Messenger RNA oder mRNA genannt wird. Sie stellten fest, dass mRNA eine große Sache ist, weil sie unseren Zellen sagt, Proteine herzustellen. Proteine sind der Beton unseres Körpers. Sie übernehmen viele wichtige Funktionen, aber die drei, die wir im Detail besprechen werden, sind ihre Fähigkeit, unsere Muskeln zu bewegen und Sauerstoff in unserem Blut zu transportieren, was uns gesund und stark hilft.
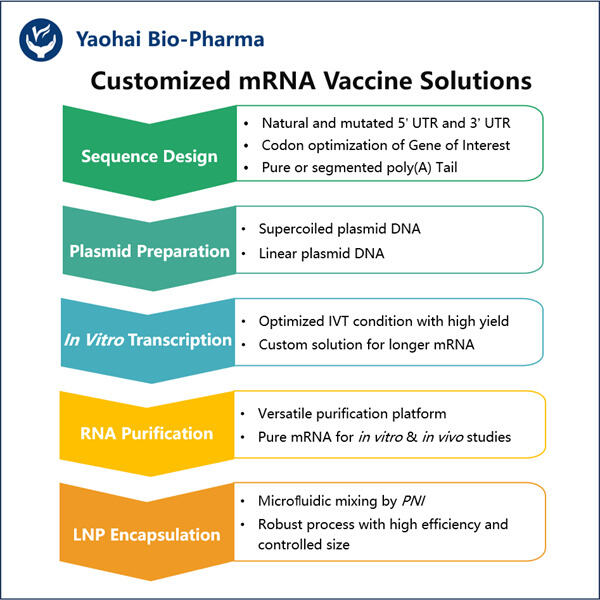
In den nächsten zehn Jahren versuchten Wissenschaftler herauszufinden, wie sie mRNA in Zellen bekommen könnten, damit diese ihre gewünschten Proteine synthetisieren, sowie die von Yaohai. E. coli Fermentation für VLP-Produktion . Es war eine lange und schwierige Reise für sie. Sie mussten entdecken, wie man das beste Skript namens mRNA herstellt, damit es nicht versehentlich etwas in unseren Körper auslöst, was uns schaden könnte.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN