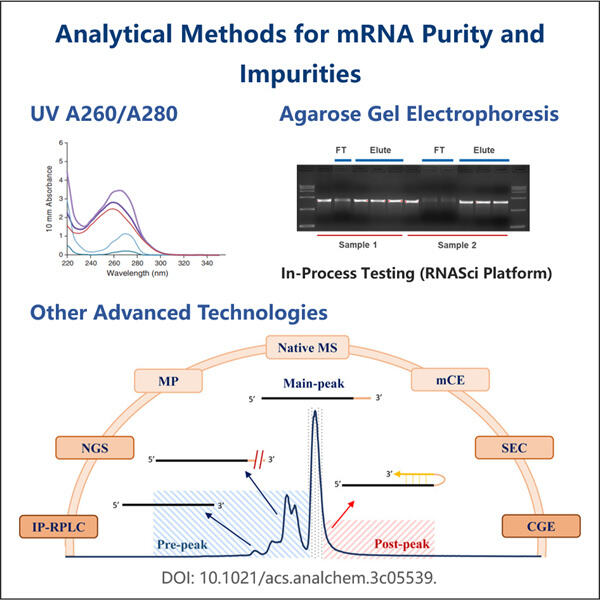
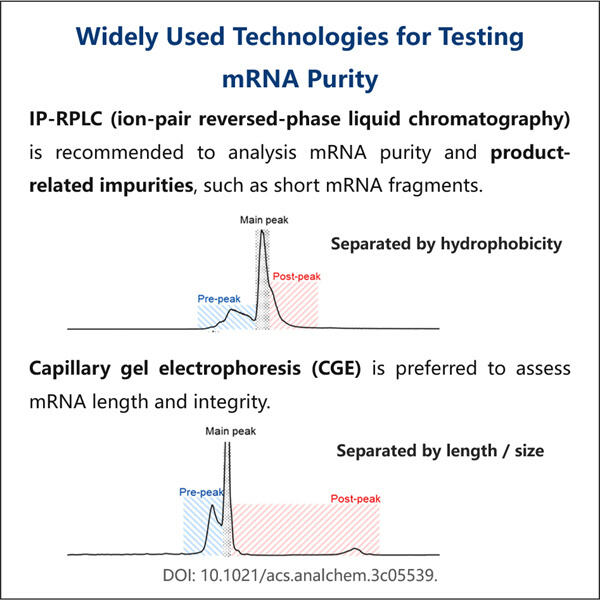
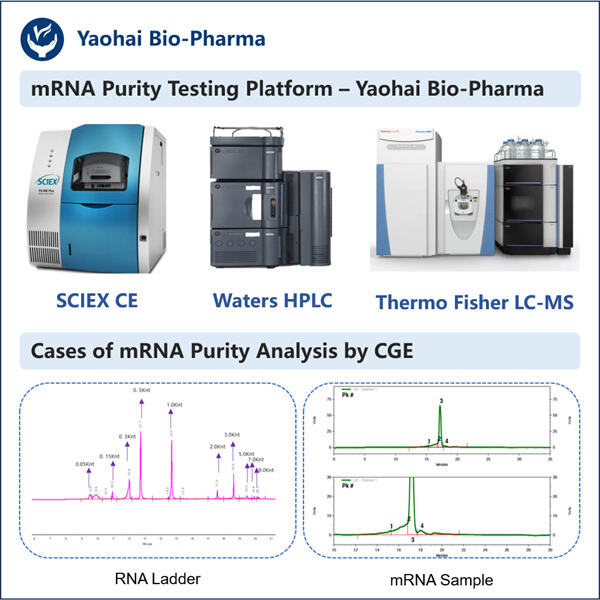
RNA-Seq-Ressourcen, um Gene zu identifizieren und neue lebensrettende Medikamente zu entwickeln, benötigen Forscher gute mRNA-Probenqualität, außerdem bietet Yaohai Produkte wie Reporter circRNA . Boten-Ribonukleinsäure (mRNA), eine Art von Molekül, das genetische Anweisungen von deiner Desoxyribonukleinsäure zu den Ribosomen transportiert. Ribosomen sind winzige Teile unserer Zellen, die bei der Produktion von Proteinen helfen, die wir benötigen, damit unser Körper richtig funktioniert. Reinige und unverfälschte mRNA-Proben sind für Forscher entscheidend, um zuverlässige Ergebnisse mit ihren Experimenten zu erzielen.
wissenschaftler verwenden mRNA-Proben in einer Vielzahl von Untersuchungen, von der Erforschung dessen, wie Gene funktionieren, bis hin zum Entwurf neuer Medikamente zum Nutzen der Menschen. Um sie korrekt zu dokumentieren, müssen sie sicherstellen, dass ihre Ergebnisse auf genauen mRNA-Proben basieren. Unnötiger Schmutz, der in die mRNA-Proben gerät, könnte ihre Experimente und Ergebnisse verfälschen, was zu falschen Ergebnissen führen kann, wenn sie diese durchführen. Gasparini gibt das Beispiel, dass selbst eine geringe Menge an ribosomaler RNA (rRNA) in einer mRNA-Probe die Daten darüber, wie Gene exprimiert und funktionieren, verfälschen könnte. Wenn dies nicht kontrolliert wird, könnten Forscher zu falschen Schlüssen aus ihren Befunden kommen.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN