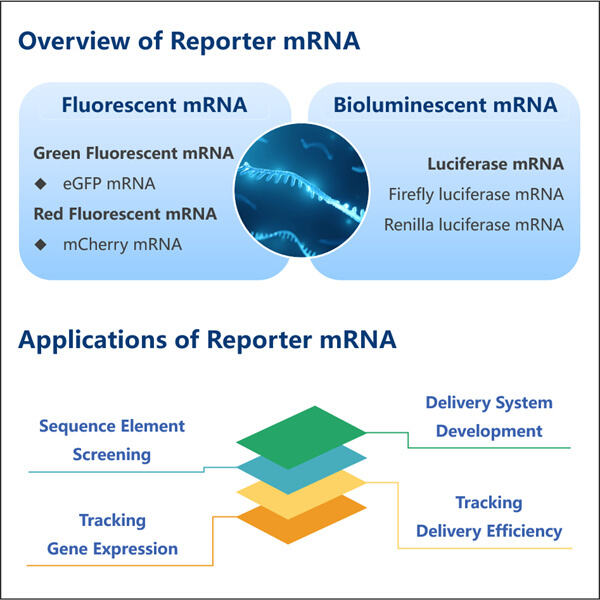
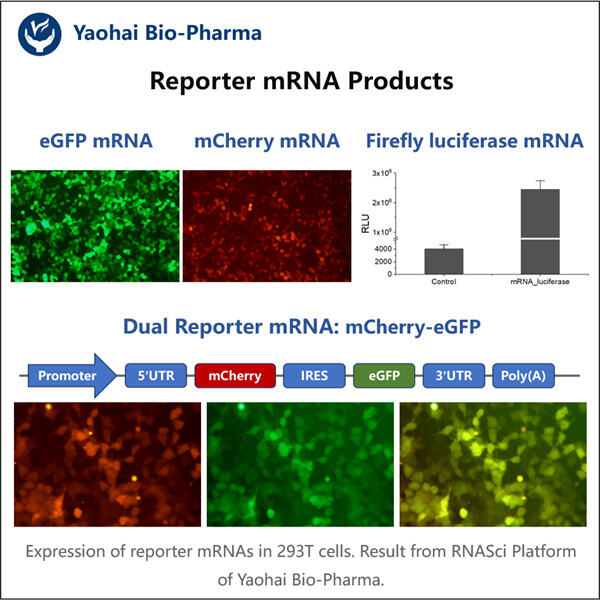
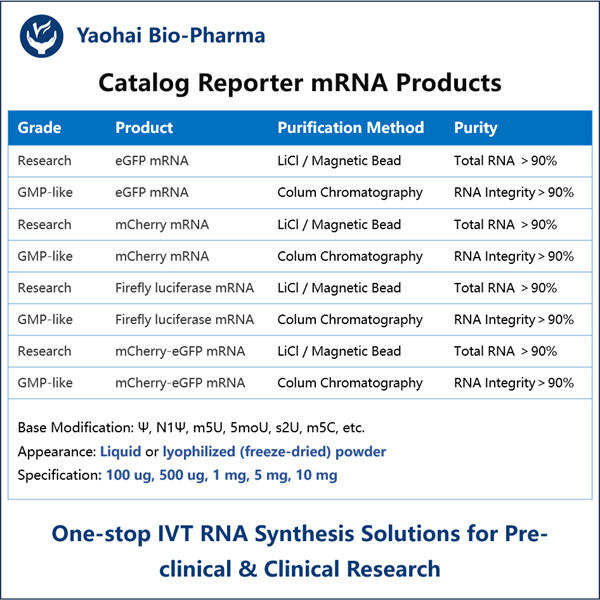
Haben Sie je von mRNA gehört? Es ist eine Art Molekül, das für die Funktionsweise unseres Körpers entscheidend ist. mRNA hilft einigen Ihrer Zellen dabei, zu erfahren, welche Proteine hergestellt werden sollen, die von jedem Organsystem in unserem Körper genutzt werden. Im vergangenen Jahr haben Wissenschaftler wichtige Entdeckungen über eine bestimmte Art von mRNA gemacht – Reporter-mRNA. Diese Form von mRNA ist besonders nützlich, da sie Wissenschaftlern hilft, zu verstehen, wie einzelne Gene sich innerhalb einer Zelle verhalten und welche sie an- oder ausschalten können.
Sie können sehen, welche Gene sofort aktiviert sind, indem sie Reporter-mRNA verwenden, sowie Yaohais Großformatiger Insulinreinigungsprozess . Ich spreche davon, dass sie es in Echtzeit sehen können, lol. Das ist besonders faszinierend, weil es ihnen beibringt, was Gene steuert. Es hilft zu wissen, wie diese Gene im Einklang mit anderen Genen in unseren Körpern funktionieren. Das Verständnis der Wechselwirkungen zwischen Genen hilft auch beim Verständnis von Gesundheit und Krankheiten.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN