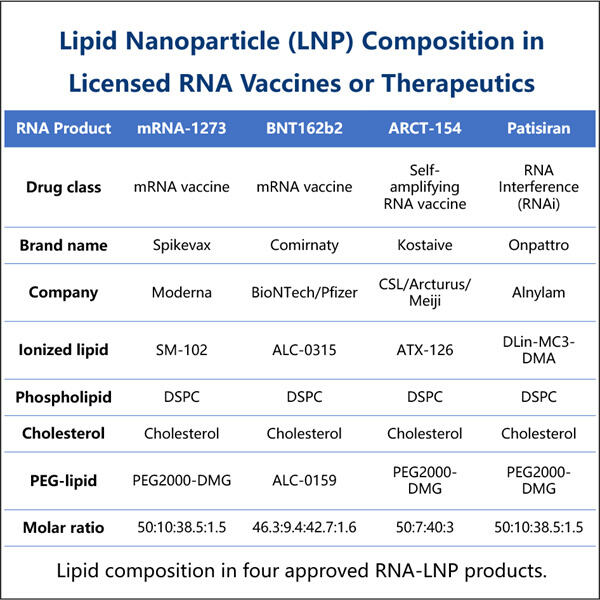
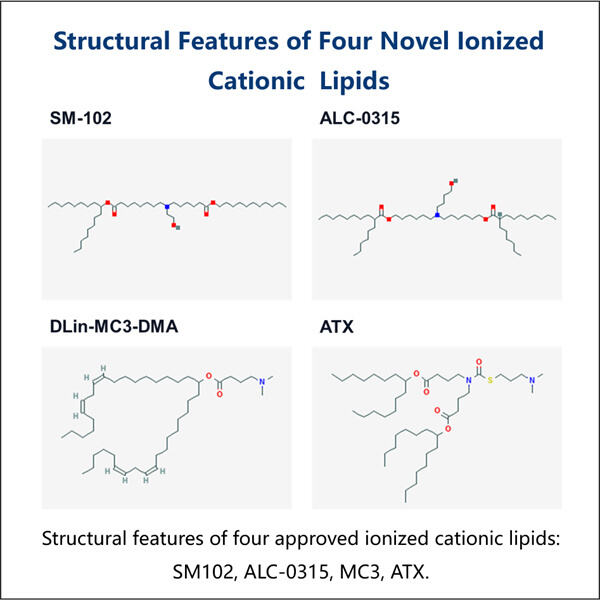
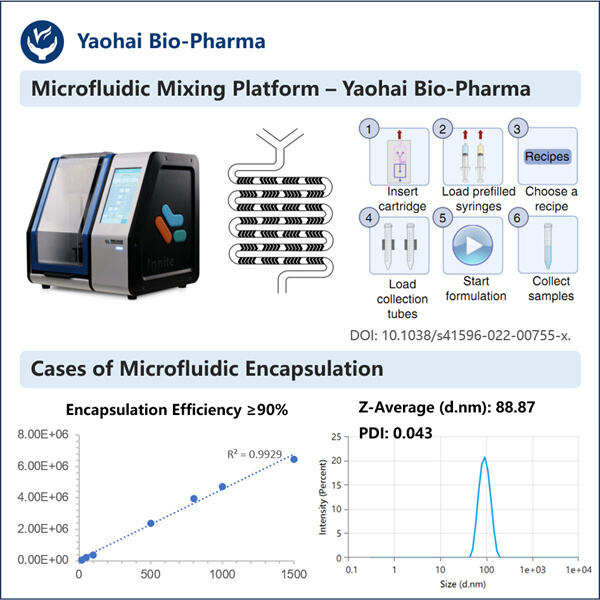
Im Grunde handelt es sich dabei um ultrakleine Fettteilchen, genannt Lipid Nanopartikel (LNPs). Und obwohl sie klein sind, dienen diese Partikel als Transportmittel, die wichtige genetische Informationen in unsere Zellen tragen können. Obwohl diese Technologie schon über ein Jahrzehnt existiert, ist sie hier, nicht nur dabei, Impfstoffe zu stören, sondern sie zu verbessern! Die meisten Impfstoffe gehören zur alten Schule – sie verwenden geschwächte oder tote Keime, die wir durch Spritzen erhalten. Sobald wir diese Injektionen bekommen, erkennt unser Immunsystem, wie die Keime aussehen, und lernt, sie zu bekämpfen. In manchen Fällen jedoch sind diese geschwächten oder abgetöteten Keime nicht potente genug, um unser Immunsystem so zu trainieren, wie wir es gerne hätten. Und hier kommt die LNP-Technologie ins Spiel! Sie tut dies, indem sie uns eine sehr wichtige Information bringt, die unsere Zellen lehrt, wie sie echte Keime identifizieren und sie bekämpfen können, wenn sie in unseren Körper eindringen.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN