تبدو الطبابة الآن مختلفة كثيرًا عما كانت عليه من قبل. بعد بدء علاجات جديدة وأصبحت أكثر كفاءة في صنع الأدوية، تم مساعدة العديد من الأفراد على التغلب على أمراضهم. ياوهاي تصنيع الحمض النووي البلازميدي GMP هي منطقة مفتاحية واحدة أحدثت تغييرات هائلة في صناعة الكيميائيات. الببتيدات هي بروتينات قصيرة يمكن أن تكون مفيدة لعلاج مجموعة متنوعة من المشاكل الصحية، على سبيل المثال، السكري والببتيدات. نحن ياوهاي ونتبع ممارسات جيدة لإنتاج هذه الببتيدات بأمان لضمان جودة المنتج.
نأخذ إنتاج الببتيدات على محمل الجد ونتبع إرشادات صارمة تضمن منتجًا آمنًا وذو جودة. أيدينا على عملية النمو الخاصة بنا، من اختيار المواد إلى قياس المنتج؛ في ياوهاي، نبذل قصارى جهدنا لاستخدام معدات تقنية عالية للحصول على أفضل الببتيدات. نستخدم فريقًا من المهندسين المؤهلين الذين يعرفون كل خطوة أثناء العملية، والتي يتم تنفيذها بدقة.
الأمان هو الأولوية القصوى في جهودنا لإنتاج الببتيدات. منتجاتنا نظيفة ومتوافقة. البكتيريا والفيروسات الرئيسية التي قد يكون لها القدرة على جعل المنتج غير صالح للاستخدام البشري، ياوهاي GMP Semaglutide Manufacturing هي تركيزنا الأساسي. نجري التجارب في غرف نظيفة حيث تكون جميع الأشياء معقمة من أي تلوث. نختبر أيضًا مقارباتنا لضمان أنها آمنة وفعالة. وللتأكد بشكل أكبر، نختبر كل دفعة من منتجاتنا لضمان مرورها بمواصفاتنا الصارمة وأنها مناسبة للاستخدام.
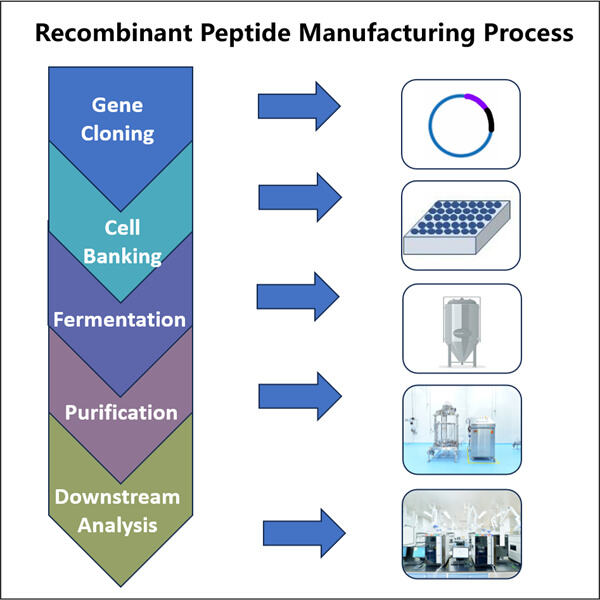
تصنيع الببتيدات المتبادلة: واحدة من الطرق الخاصة التي نستخدمها لإنشاء الببتيدات تُسمى تصنيع الببتيدات المتبادلة. نعتمد بالتالي على التعبير عن البروتينات рекомбинانت للحصول على هذه البروتينات القيمة. هناك بعض المعدات المتخصصة والمعرفة اللازمة للتغلب على نوع التنظيف الذي تحتاجه، كما يجب الحفاظ على السلامة أيضًا. قد اكتسبنا خبرة كبيرة في هذا المجال في ياوهاي. وبالفعل، نلتزم بالمعايير الأفضل لضمان أن تكون جميع منتجاتنا ذات جودة عالية وآمنة تمامًا للاستخدام.

ومع زيادة عدد الحالات الاستخدامية، يحتاج المزيد والمزيد من الناس إلى الببتيدات العلاجية. إذا أردنا تلبية الطلب عليهم، يجب أن نتمكن من إنتاج الآلاف من الأطنان من الببتيدات دون أي انخفاض في الجودة / وعدم المساس بالأمان. وهذا هو المكان الذي تدخل فيه تصنيع GMP. ياوهاي إنتاج VHH مضاد CD8 وفقًا لنظام GMP هي اختصار لمعايير التصنيع الجيدة، وتجعل إجراءاتنا لتصنيع الببتيدات قياسية. يمكننا تركيب كميات كبيرة من الببتيدات في ياوهاي ولكننا دائمًا نحافظ على سلامتها وجودتها العالية. سيتلقى المرضى أفضل رعاية في قضايا صحتهم الفردية.
ياوهاي بيو-فارما، وهي واحدة من أكبر 10 مصنّعي المنتجات البيولوجية، تخصصت في التخمير المجهرى. لقد أنشأنا منشأة تصنيع ببتيدات إعادة التركيب وفق معايير GMP مع قدرات قوية في البحث والتطوير ومرافق تصنيع متقدمة. هناك خمس خطوط لإنتاج المواد الخام تتوافق مع معايير GMP لتنقية وتخمير الخلايا الدقيقة، بالإضافة إلى خطين لتعبئة الأدوية في الزجاجات والكروتيدات وكذلك الإبر المسبقة التعبئة. تتاح أحجام التخمير بما في ذلك 100لتر، 500لتر، 1000لتر و2000لتر. مواصفات تعبئة الزجاجات تتراوح بين 1مل - 25مل. أما مواصفات تعبئة الكروتيدات أو السرنجات المسبقة التعبئة فتتراوح بين 1-3مل. ورشة الإنتاج متوافقة مع cGMP وتقدم إمداداً مستقراً بالمنتجات التجارية والعينات السريرية. منشأتنا تنتج الجزيئات الكبيرة التي يتم شحنها حول العالم.
ياوهاي بايو-فارما، وهي شركة رائدة في مجال خدمات CDMOs لأدوية الميكروبات الحيوية، تقع في جيانغسو. نحن نركز على العلاجات واللقاحات المنتجة ميكروبيًا والتي تتضمن تصنيع الببتيدات المُعاد تركيبها وفق معايير GMP لصحة الإنسان، البيطرية وكذلك إدارة صحة الحيوانات الأليفة. لدينا منصات RD الأكثر تقدمًا والتكنولوجيا المتطورة التي تغطي العملية الإنتاجية بأكملها، بدءًا من تطوير السلالات الدقيقة، وبنوك الخلايا، وتطوير العمليات والطرق وحتى التصنيع السريري والتجاري، مما يضمن إنتاج حلول جديدة بنجاح. قدمنا خبرة واسعة في معالجة الخلايا الدقيقة الحيوية. تم إكمال أكثر من 200 مشروع بنجاح، وندعم عملاءنا في تجاوز اللوائح مثل تلك الخاصة بالهيئة الأمريكية للرقابة على الغذاء والدواء (FDA) والهيئة الأوروبية للأدوية (EMA). كما نساعد العملاء مع الهيئة الأسترالية TGA وهيئة NMPA الصينية. تمكننا خبرتنا والمعرفة المهنية الواسعة من الاستجابة السريعة لمتطلبات السوق وتقديم خدمات CDMO مخصصة.
ياوهاي بيوفارما هي واحدة من أكبر 10 شركات CDMO الميكروبية التي تدمج إدارة الجودة والقضايا التنظيمية. لقد طورنا نظامًا قويًا لإدارة الجودة يتماشى مع معايير GMP الحالية واللوائح العالمية. فريقنا التنظيمي لديه فهم عميق للإطارات التنظيمية العالمية. هذا يمكّننا من تسريع إطلاق المنتجات البيولوجية. نحن نضمن عمليات إنتاج قابلة للتتبع وكذلك منتجات ذات جودة عالية ومتصلة بالمبادئ التوجيهية لـ FDA الأمريكية وEMA الأوروبية. كما أن تصنيع الببتيدات المُعاد تركيبها وفقًا لمعايير GMP وNMPA الصينية يتم الالتزام بها أيضًا. قدّمت ياوهاي بيوفارما بنجاح تدقيقًا ميدانيًا أجرته شخصية مؤهلة معتمدة من الاتحاد الأوروبي (QP) لمراجعة نظام GMP ومرافق الإنتاج لدينا. كما مررنا أيضًا بعمليات التصديق الأولى لأنظمة إدارة الجودة ISO9001 وأنظمة إدارة البيئة ISO14001.
لدى Yaohai Bio-Pharma خبرة في تصنيع الأدوية البيولوجية التي تُنتج من الميكروبات. نقدم حلول RD المخصصة وخدمات التصنيع مع تقليل المخاطر المحتملة. عملنا باستخدام تقنيات متنوعة مثل الوحدات الخلوية рекombinat، اللقاحات (بما في ذلك الببتيدات)، عوامل النمو، الهرمونات، وتصنيع الببتيدات рекombinat وفقًا لمعايير GMP. نحن متخصصون في العديد من الكائنات الدقيقة مثل الإفراز الخلوي والخارجي للخميرة (تنتج حتى 15 جم/لتر) والإفراز الداخلي، والبكتيريا القابلة للذوبان داخل الخلية وجسم الشمول (تنتج حتى 10 جم/لتر). كما طورنا منصة تخمير BSL-2 لإنشاء لقاحات بكتيرية. لدينا سجل حافل في تحسين عمليات الإنتاج، مما يزيد من العائدات ويقلل من التكاليف. لدينا فريق تقني كفؤ للغاية لضمان تسليم المشاريع في الوقت المحدد وبجودة عالية. هذا يساعدنا على إدخال منتجاتكم الفريدة إلى السوق بشكل أسرع.