ياوهاي هو مصنع للسيتوكينات أو البروتينات الخاصة. هذه السيتوكينات هي العناصر الرئيسية لصحة الجهاز المناعي. ولكي يتم اعتبار هذه البروتينات آمنة ومناسبة للمستشفيات والعيادات، يجب أن يلتزم ياوهاي أيضًا بالقوانين الصارمة التي تم وضعها كممارسات تصنيع جيدة أو GMP. إنهم يحرصون على فحص السيتوكينات بشكل جيد لضمان خلوها من أي شوائب أو مواد ضارة. لذلك، قبل استخدام هذه البروتينات، يجب أن تخضع لفحص شامل لتأكيد أنها تم تنقيتها بشكل صحيح وهي آمنة للاستخدام على المرضى.
يستخدم ياوهاي تقنية متقدمة رائدة على المستوى العالمي لجعل جودة السيتوكينات الخاصة بهم تطابق تلك التي يمكنك العثور عليها في أماكن أخرى. مزودون بالمعدات التي تمكنهم من تشكيل السيتوكينات الخام إلى عوامل شفائية نقية وفعّالة يمكن للأشخاص الذين يحتاجون إليها الحصول عليها. باستخدام هذه الآلات والعمليات المتقدمة، يمكنهم تصنيع ياوهاي المصفى بدقة. GMP GLP-1GIP Tirzepatide API التي تحتاج إلى وصفها طبياً. يقدمون السيتوكينات التي تعتبر أساسية لتحسين صحة المريض، وبشكل أساسي، هذه التقنية رائعة للغاية.
يجب أن تُنتج السيتوكينات بواسطة ياوهاي في أمان مراحل الحياة كما حددتها المنظمات الصحية في جميع أنحاء العالم لضمان أنها آمنة للاستخدام البشري. بسبب هذا التنظيم، فقد ضمنت ياوهاي أن كل دفعة من السيتوكينات يتم فحصها وإجراء الفحوصات اللازمة عليها بشكل صارم. ولذلك يمكن للمرضى التأكد من أن منتجات ياوهاي تصنيع الحمض النووي البلازميدي GMP آمنة وفعّالة لعلاجهم.
لذلك، طورت ياوهاي تقنيات جديدة لإنتاج سيتوكينات ذات جودة عالية بتكلفة أقل. لديهم طرق ذكية للغاية لإنتاج سيتوكينات نقية وقوية. وهذا مهم لأن استجابة قوية للسيتوكينات المناعية قد تساعد المريض على التعافي بشكل أفضل. تسعى كوستار بايو إلى إنتاج سيتوكينات عالية الجودة لأولئك الذين يحتاجون إليها، وتهدف الشركة دائمًا إلى تحسين عملياتها.
طورت شركة Yaohai عملية لإنتاج السيتوكينات بسرعة وموثوقية. يمكنهم إنتاج هذه السيتوكينات بسرعة كبيرة، مما يجعلها متاحة للمستشفيات والعيادات. يجب تسليم البروتينات بسرعة؛ وهذا يسمح للمرضى بتلقي العلاج الذي يحتاجون إليه دون انتظار. من خلال وجود عملية إنتاج موثوقة، تضمن Yoahai توفر السيتوكينات دائمًا للمرضى الذين يحتاجون إليها.
عندما نقوم بإنتاج السيتوكينات، فمن الضروري جدًا تحقيق أعلى مستوى ممكن من الجودة. لديهم إرشادات صارمة للغاية لضمان بقاء السيتوكينات نقية وغير ملوثة بأي مادة سامة (وجود مذيبات عضوية قد ت intoxicate الخلايا). يتم اختبار هذه السيتوكينات عدة مرات لضمان السلامة والوظيفية. حتى بعد تنقية المادة الخام، يجب أن تمر كل سيتوكين عبر عدة خطوات للتحكم في الجودة لضمان أنها آمنة بما يكفي للاستخدام في المستشفيات أو العيادات.
خلال هذا العملية، يستخدم ياوهاي أدوات بحث حديثة لإنتاج السيتوكينات. هذه هي الأدوات المستخدمة في اختبار وعزل خصائص السيتوكينات المهمة للقمع مع الحفاظ على أعلى نشاط. ياوهاي GMP Semaglutide Manufacturing باستخدام التكنولوجيا المتقدمة، يمكن إنتاج كميات كبيرة من السيتوكينات بسرعة وكفاءة وجودة عالية. في الطب، تظهر هذه الكفاءة فيما يتعلق بالسيتوكينات.
لدى Yaohai Bio-Pharma خبرة في تصنيع الأدوية البيولوجية المصنوعة من الميكروبات. نقدم حلول RD مخصصة وخدمات التصنيع مع تقليل المخاطر إلى الحد الأدنى. عملنا مع أنماط متنوعة مثل اللقاحات الفرعية рекombinatnaya، الببتيدات، الهرمونات، السيتوكينات، عوامل النمو، الأجسام المضادة ذات المجال الواحد، الإنزيمات، الحمض النووي البلازميدي، mRNA وغيرها. تخصصنا في عدة ميكروبات مثلخميرة الإفراز الخلوي والداخلي (الإنتاج يصل إلى 15 جم/لتر)، البكتيريا الذائبة داخل الخلايا وأجسام الشمول (الإنتاج يصل إلى 10 جم/لتر). كما قمنا بإنشاء نظام تخمير BSL-2 لإنتاج لقاحات السيتوكينات GMP. نحن خبراء في تحسين عمليات الإنتاج، زيادة الإنتاج وتقليل التكاليف. لدينا فريق تقني كفؤ يضمن تسليم المشاريع في الوقت المحدد وبجودة عالية. هذا يسمح لنا بتقديم منتجاتكم الفريدة بشكل أسرع إلى السوق.
ياوهاي بيو-فارما، الرائدة في مجال خدمات CDMO الخاصة بالأدوية الحيوية الدقيقة، مقرها في جيانغسو. ركزنا على الأدوية واللقاحات المنتجة بواسطة الكائنات الدقيقة التي تتناسب مع الاستخدام البشري، البيطري وإدارة تصنيع السيتوكينات وفقًا لمعايير GMP. نمتلك أكثر التقنيات المتقدمة في البحث والتطوير وكذلك منصات التصنيع التي تغطي العملية بأكملها من هندسة سلالات الكائنات الدقيقة، بنوك الخلايا، تطوير العمليات والطرق إلى التصنيع التجاري والسريري، مما يضمن توفير الحلول الأكثر تقدمًا بنجاح. تراكم لدينا خبرة كبيرة في معالجة الكائنات الدقيقة الحيوية. قدمنا أكثر من 200 مشروع حول العالم وساعدنا عملائنا في التعامل مع القوانين واللوائح الخاصة بالهيئة الأمريكية للرقابة على الأغذية والأدوية (FDA)، وكالة الأدوية الأوروبية (EMA)، وهيئة تنظيم الأدوية الأسترالية (TGA)، والهيئة الوطنية لإدارة المنتجات الطبية في الصين (NMPA). المعرفة المتخصصة والخبرة الواسعة تمكننا من التكيف بسرعة مع احتياجات السوق وتقديم خدمات CDMO مخصصة.
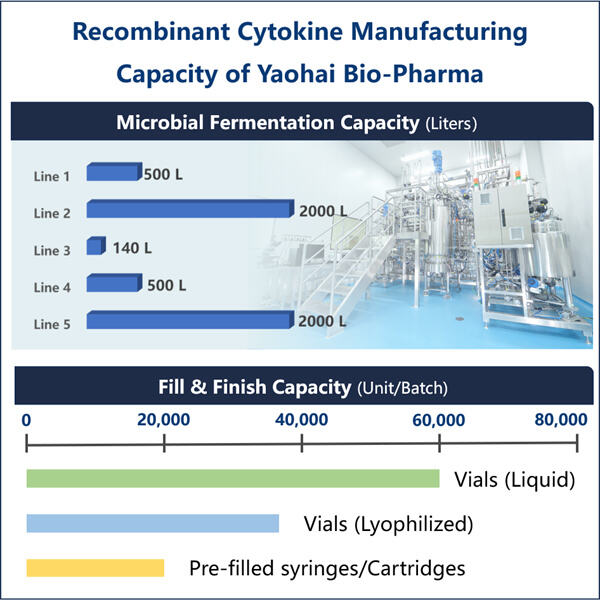
ياوهاي بيو-فارما، وهي واحدة من أكبر 10 مصنّعي المنتجات البيولوجية، متخصصة في التخمير المجهرى. لقد أنشأنا مصنعًا فعالاً يحتوى على مرافق متقدمة وقدرات قوية في البحث والتطوير والإنتاج. هناك خمس خطوط إنتاج مواد دوائية تتوافق مع معايير GMP للتخمير والتنقية المجهرية، بالإضافة إلى خطين لتعبئة الأدوية النهائية في الزجاجات والأمبولات والحقن المسبقة التعبئة. تتراوح أحجام عمليات التخمير بين 100 لتر وإنتاج السيتوكينات حسب معايير GMP. مواصفات التعبئة للزجاجات هي من 1 مل إلى 25 مل. أما بالنسبة للتعبئة في الأنبوب أو الإبرة المسبقة التعبئة فهي تتراوح بين 1-3 مل. ورشة الإنتاج متوافقة مع معايير cGMP وتضمن توفير العينات السريرية والتجارية بشكل مستمر. منشأتنا تنتج الجزيئات الكبيرة التي يتم شحنها عالميًا.
ياوهاي بيوفارما، واحدة من أكبر 10 شركات CDMO المجهرية التي تدمج إدارة الجودة والشؤون التنظيمية. نظام جودتنا متوافق مع معايير GMP الحالية واللوائح الدولية. فريق خبراء الشؤون التنظيمية لدينا متمرس في الإطارات التنظيمية العالمية لتسريع إطلاق المنتجات البيولوجية. نحن نضمن إجراءات إنتاج قابلة للتعقب، منتجات ذات جودة عالية، وكذلك الامتثال لمتطلبات تصنيع السيتوكين GMP ووكالة الأدوية الأوروبية (EMA). كما يتم الالتزام بمتطلبات وكالة الأدوية الأسترالية (TGA) وإدارة الأدوية والمنتجات الطبية الوطنية الصينية (NMPA). لقد نجحت ياوهاي بيوفارما في اجتياز التدقيق الشخصي الذي أجرته شخصية مؤهلة من الاتحاد الأوروبي (QP) لفحص نظام GMP ومرافق الإنتاج لدينا. كما أننا أكملنا عمليات التدقيق الأولية لنظام إدارة الجودة ISO9001 ونظام إدارة البيئة ISO14001.