مAnalogue GLP-2 دواء جديد يجعل علماء ياوهاي متحمسين جدًا. دواء له القدرة على مساعدة الأشخاص المرضى - أولئك الذين يعانون من مرض كرون ومتلازمة الأمعاء القصيرة. عدم القدرة على الحصول على العناصر الغذائية الأساسية من نظامهم الغذائي اليومي واضح جدًا مع هذه الأمراض. أولئك تصنيع لقاح الحمض النووي الريبي لا يوجد مهرب من الفيتامينات والمعادن الموجودة في طعامهم سوى محاولة عديمة الجدوى لإيجاد طريق إلى الهيكل الذي هو جسمهم. وثم يمكن لمركب GLP-2 المماثل أن ينقذ الموقف عن طريق تسهيل امتصاص العناصر الغذائية في أجسامهم، ومن ثم جعلهم يشعرون بتحسن، وتقديم جودة حياة أفضل.
لم يكن إنتاج الدواء أمرًا سهلاً. إذا كان دواء خاصًا، فإنه يحتاج إلى رعاية خاصة وحساسة لإنشائه. هذا هو ما يُعرف بالتصنيع البيولوجي. لتوضيح الأمر، التصنيع البيولوجي هو تخمير الإي كولاي لإنتاج جسيمات VLP عملية تحفيز الخلايا الحية والكائنات الدقيقة لإنتاج الأدوية. بينما يتم تصنيع معظم الأدوية عن طريق مزج مواد كيميائية مختلفة، فإن هذا يعطي طريقة جديدة للمетод التقليدي. لأن التصنيع البيولوجي أكثر طبيعة، باستخدام الكائنات الحية لإنتاج الأدوية قد يكون أكثر أمانًا وسهولة على المرضى.
هناك العديد من الخطوات المهمة التي يجب على العلماء اتخاذها لإنتاج مركب GLP-2 المماثل. كل شيء يبدأ بتعديل الجينات. هذا يسمح لهم باستقطاب الجينات الصديقة للإنسان من أي كائن حي على الأرض، شريطة ألا تكون ملوثة بأي شكل من الأشكال. تم تحقيق ذلك من خلال تعديل الجينات لجعل بعض الخلايا تنتج الدواء. هذه الخلايا المهندسة تصنيع VLP لفيروس بكتيري Q ثم تُزرع في أحواض تُعرف باسم مفاعلات الأحياء. ما يجعل هذه المفاعلات فريدة هو السوائل داخلها التي تقدم العناصر الغذائية اللازمة لخلايا لتكون قوية وصحية أيضًا. يستغرق الأمر وقتًا طويلاً لنمو عدد كافٍ من الخلايا لإنتاج دفعة من محاكي GLP-2، وجعل الدواء فعالاً مهم جدًا.
بعد أن تنمو الخلايا بشكل كافٍ، يمكن للعلماء حصاد الدواء. يتم استخراج محاكي GLP-2 بعناية بواسطة آلات خاصة من الخلايا. بمجرد استخراج الدواء، يمر بعدة اختبارات لتأكيد أنه آمن للاستخدام وأنه يعمل بشكل صحيح لمساعدة المرضى. وهذا تصنيع لقاحات VLP المرتبطة يُفترض أن يتم فحصه بدقة ليكون مقبولًا بصريًا، ثم ينتقل إلى التغليف. بعد ذلك، يتم إرسالها للأطباء لتوزيع الدواء مباشرة على الأشخاص الذين يحتاجون إلى تحسين صحتهم.
يمكن للعلماء استخدام التصنيع البيولوجي لإنتاج مAnalogue GLP-2 بعدة طرق أخرى. دائمًا يعمل العلماء على تحسين وتطوير هذه التقنيات، والعلماء في ياوهاي يعملون بجد. تصنيع محفز GLP-1 يريدون تصنيع الدواء بشكل أسرع وبتكلفة أقل لجعله متاحًا أكثر للسكان. أحد النهج المثيرة التي يقومون بدراستها هو التصنيع البيولوجي المستمر. يتم ذلك باستخدام حجم أصغر من المفاعل الحيوي الذي يمكّن الخلايا من إنتاج الدواء بشكل مستمر. مما يعني أن العلماء قادرون على إنتاج كميات أكبر من الدواء في وقت أقل وبأقل خسارة، مما يساهم في كفاءة معالجة الشعاع الإلكتروني.
ياوهاي بيولوجي فارما هي رائدة في مجال تصنيع الأدوية البيولوجية لمشتقات GLP-2. تركيزنا الأساسي كان على إنتاج اللقاحات والعلاجات الدقيقة لعلاج الحيوانات الأليفة، والصحة البشرية والبيطرية. نحن نمتلك تقنيات متقدمة للبحث والتطوير والتصنيع تغطي العملية الإنتاجية بأكملها، من هندسة السلالات الدقيقة إلى معالجة بنوك الخلايا وتصميم الطرق، وحتى التصنيع السريري والتجاري، مما يضمن لنا تقديم الحلول الأكثر تقدمًا بنجاح. لقد جمعنا كمية كبيرة من المعرفة في مجال معالجة الكائنات الدقيقة البيولوجية. تم تنفيذ أكثر من 200 مشروع بنجاح، ونساعد عملائنا على الامتثال للوائح مثل تلك الخاصة بالهيئة الأمريكية للأدوية والغذاء (FDA) وكذلك الهيئة الأوروبية للأدوية (EMA). كما نساعد العملاء في التعامل مع الهيئة الأسترالية لتقييم الأدوية (TGA) والهيئة الوطنية لإدارة المنتجات الطبية في الصين (NMPA). بفضل خبرتنا ومهارتنا، يمكننا الاستجابة بسرعة لمتطلبات السوق وتقديم خدمات CDMO مخصصة.
يتمتع Yaohai Bio-Pharma بخبرة واسعة في تطوير الأدوية البيولوجية المستخلصة من الميكروبات. نقدم حلولًا مخصصة للبحث والتطوير وكذلك التصنيع، مع التأكد من عدم وجود أي مخاطر. عملنا على أنماط متنوعة مثل اللقاحات рекombinانت القائمة على الوحدات الفرعية، وتصنيع GLP-2 Analogue Biomanufacturing، والسيتوكينات، والعوامل النمو، والمضادات الجسدية ذات المجال الواحد، والأنزيمات، والDNA البلازميدي، والمRNA وغيرها. نحن خبراء في مجموعة متنوعة من الكائنات الدقيقة، بما في ذلك إفراز الخلايا الداخلية والخارجية لخميرة (مع إنتاج يصل إلى 15 جم/لتر) وكذلك البكتيريا القابلة للذوبان داخل الخلية وجسم الشمولية (مع إنتاج يصل إلى 10 جم/لتر). كما طورنا منصة التخمير BSL-2 لإنشاء لقاحات قائم على البكتيريا. لدينا سجل حافل في تحسين العمليات الإنتاجية، مما يؤدي إلى زيادة الإنتاج وتقليل التكاليف. ومع فريق تقني كفؤ للغاية، نضمن تسليم المشاريع في الوقت المحدد وبجودة عالية، مما يساعد على إدخال منتجاتكم إلى السوق بشكل أسرع.
ياوهاي بايو فارما هي واحدة من أكبر 10 شركات CDMO الدقيقة التي تدمج إدارة الجودة والشؤون التنظيمية. لدينا نظام لإدارة الجودة يتماشى مع المعايير الحالية لـ GLP-2 وتصنيع البيوفارما وفقًا للوائح العالمية. فريقنا التنظيمي على دراية بالإطارات التنظيمية العالمية التي تسهم في تسريع إطلاق المنتجات البيولوجية. نحن نضمن إجراءات إنتاج قابلة للتتبع، وجودة المنتجات، وكذلك الامتثال لإرشادات FDA الأمريكية وEMA الأوروبية. كما أن TGA الأسترالية وNMPA الصينية متوافقة أيضًا. لقد نجحت ياوهاي بايو فارما في اجتياز التدقيق الميداني الذي أجراه الشخص المؤهل (QP) من الاتحاد الأوروبي لنظامنا الخاص بجودة GMP وموقع الإنتاج. كما مررنا بنجاح أول عمليات التدقيق للحصول على شهادات نظام إدارة الجودة ISO9001 ونظام إدارة البيئة ISO14001.
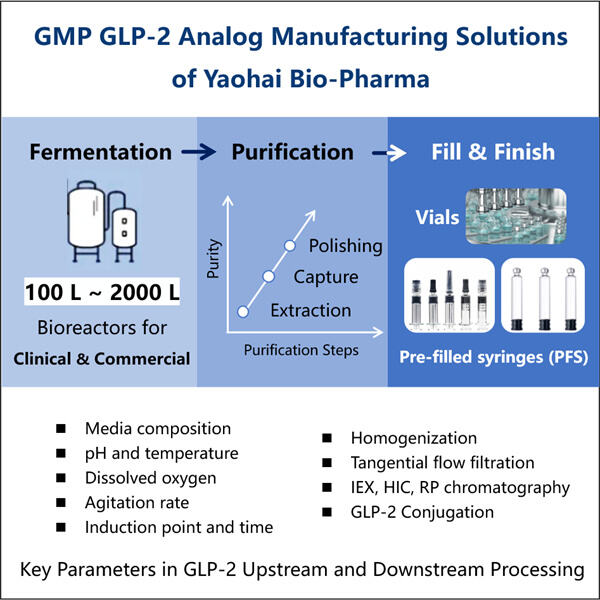
ياوهاي بيو-فارما، وهي شركة تصنيع منتجات بيولوجية لتصنيع مشتقات GLP-2، متخصصة في التخمير المجهرية. لقد أنشأنا مصنعًا حديثًا مزودًا بأحدث المعدات وقوة بحث وتطوير قوية وقدرات تصنيعية. هناك خمس خطوط إنتاج مواد دوائية تتوافق مع معايير GMP للتنقية والتخمير المجهرية، بالإضافة إلى خطين لإغلاق العبوة للأمبولات والكروتيدات والإبر المسبقة الملء. تتراوح أحجام التخمير المتاحة من 100 لتر إلى 500 لتر، 1000 لتر، وحتى 2000 لتر. تتراوح أحجام الملء من 1 مل إلى 25 مل. يتم ملء الإبر أو الكروتيدات المسبقة الملء بما يعادل 1-3 مل. يضمن مصنع الإنتاج الخاص بنا الذي يتوافق مع cGMP توفر عينات سريرية مستقرة ومنتجات تجارية. يمكن تسليم الجزيئات الكبيرة المنتجة في مصنعنا دوليًا.