Pembuatan Bank Sel Mikroba
Bank Sel Mikroba adalah mikroba hidup yang disimpan dalam tabung kecil/vial. Repositori ini dapat menampung berbagai macam bakteri, jamur, atau virus, tergantung pada minat ilmiah. Mereka meletakkan mikroba-mikroba tersebut dalam kaldu nutrisi dan kondisi khusus lainnya yang membantu menjaga mereka tetap sehat. Agar hal itu terjadi, banyak hal yang harus berjalan dengan baik bagi para ilmuwan. Mereka mempertimbangkan hal-hal seperti suhu (seberapa hangat atau dingin), kelembapan (seberapa basah atau kering udara), jumlah oksigen yang tersedia bagi mikroba ini untuk bernapas, bahkan variasi kecil dalam pH (yang menunjukkan apakah sesuatu bersifat asam atau basa).
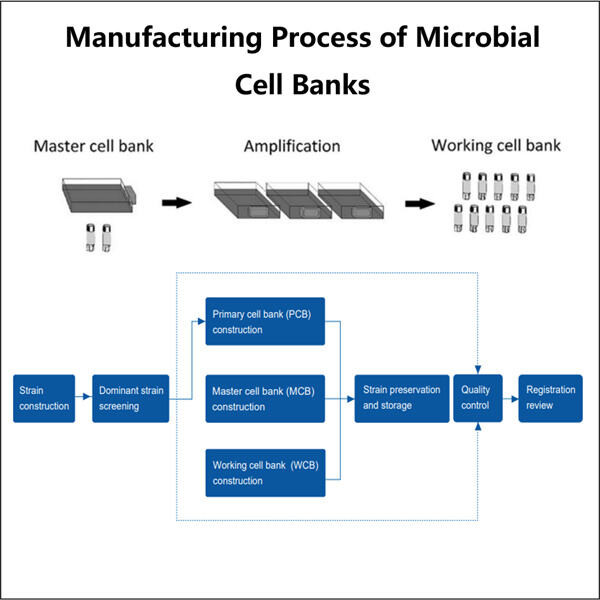
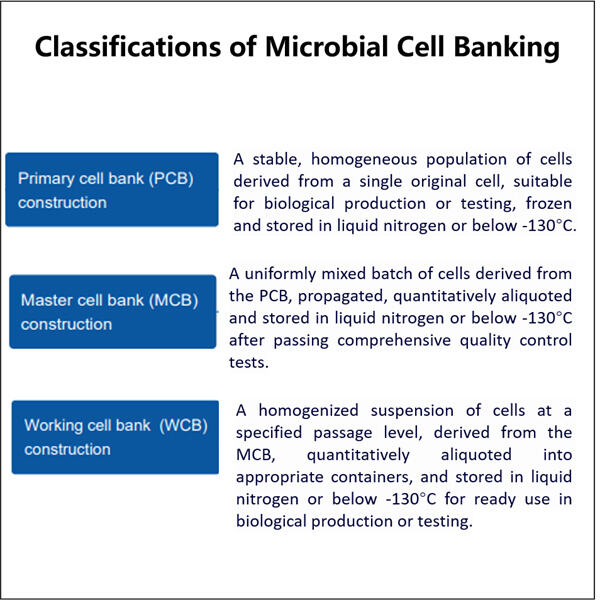
Proses Biopharmasi Mikroba Skala U seperti disebutkan di atas, sebuah bank sel mikroba dimulai dengan penemuan mikroorganisme dari lingkungannya yang alami. Ini sering melibatkan pengambilan spesimen dari lokasi seperti tanah, air, atau area lain yang menjadi tempat tinggal mikroba tersebut. Sampel-sampel ini harus dikumpulkan dengan hati-hati untuk memastikan bahwa jenis mikroba yang ditemukan akurat. Ilmuwan dapat menemukan dan mengumpulkan mikroba, lalu membawanya kembali ke laboratorium untuk dikembangbiakkan. Makanan, atau lebih tepatnya media dan kondisi khusus digunakan untuk menumbuhkan mikroba di laboratorium. Dengan kata lain, proses tersebut sangat penting — tanpanya ilmuwan tidak dapat memperoleh cukup jumlah mikroba tertentu untuk dipelajari. Ketika mikroba telah tumbuh hingga ukuran yang cukup, ia disimpan di bank sel.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN