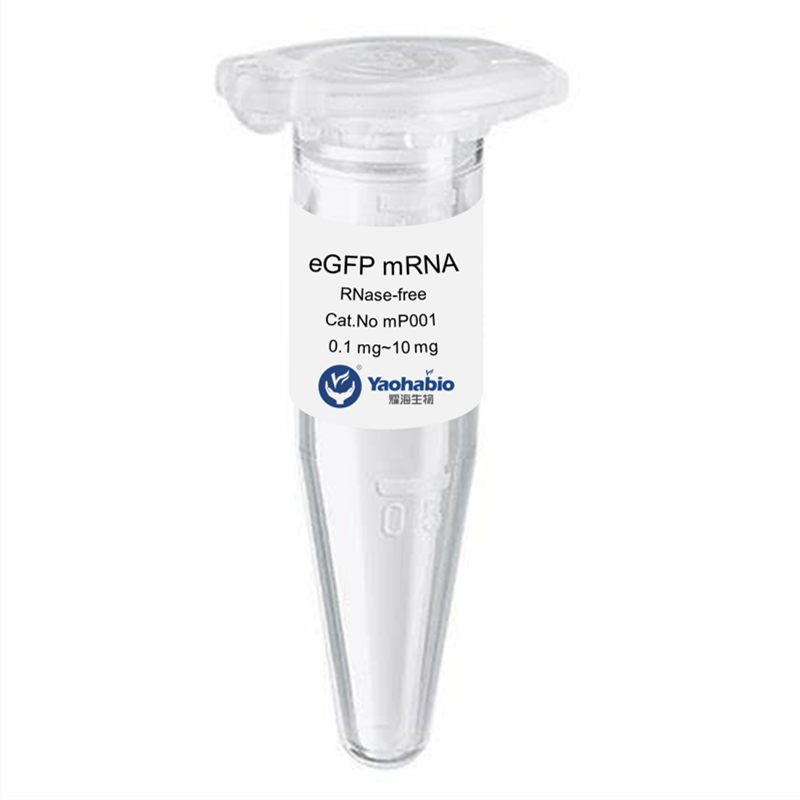
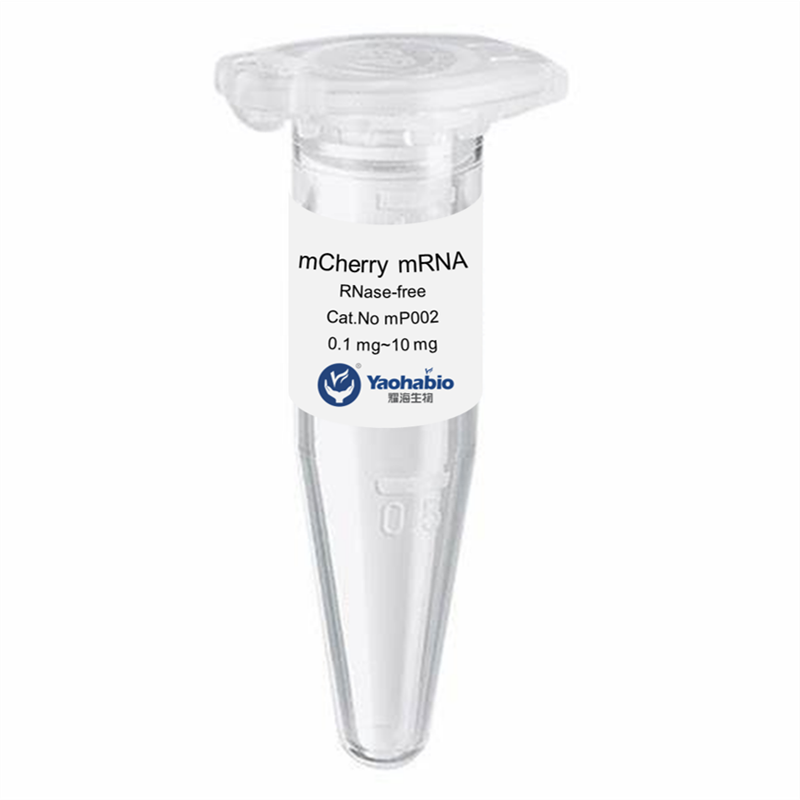
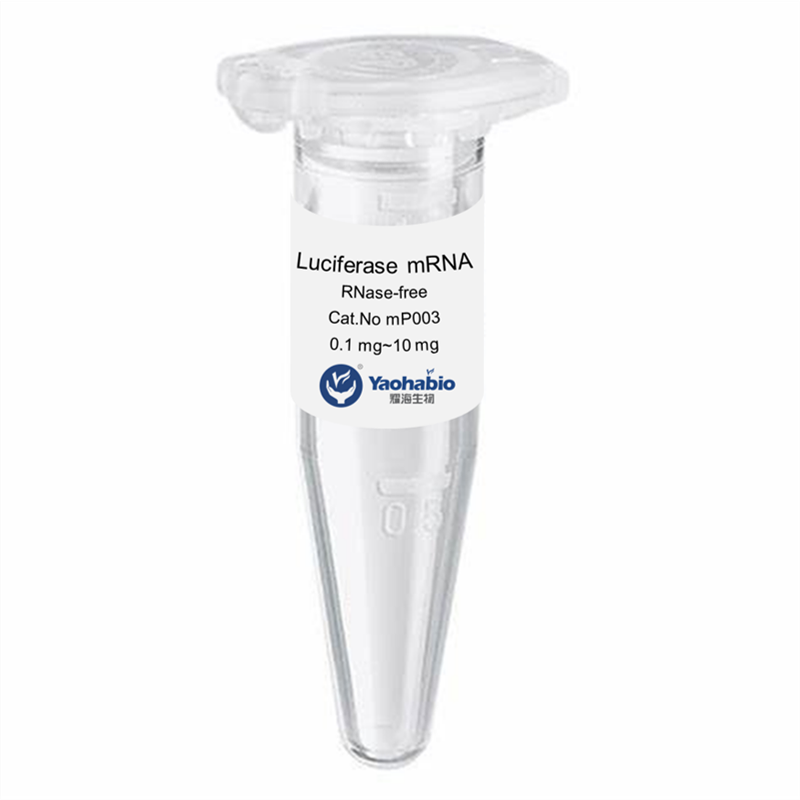
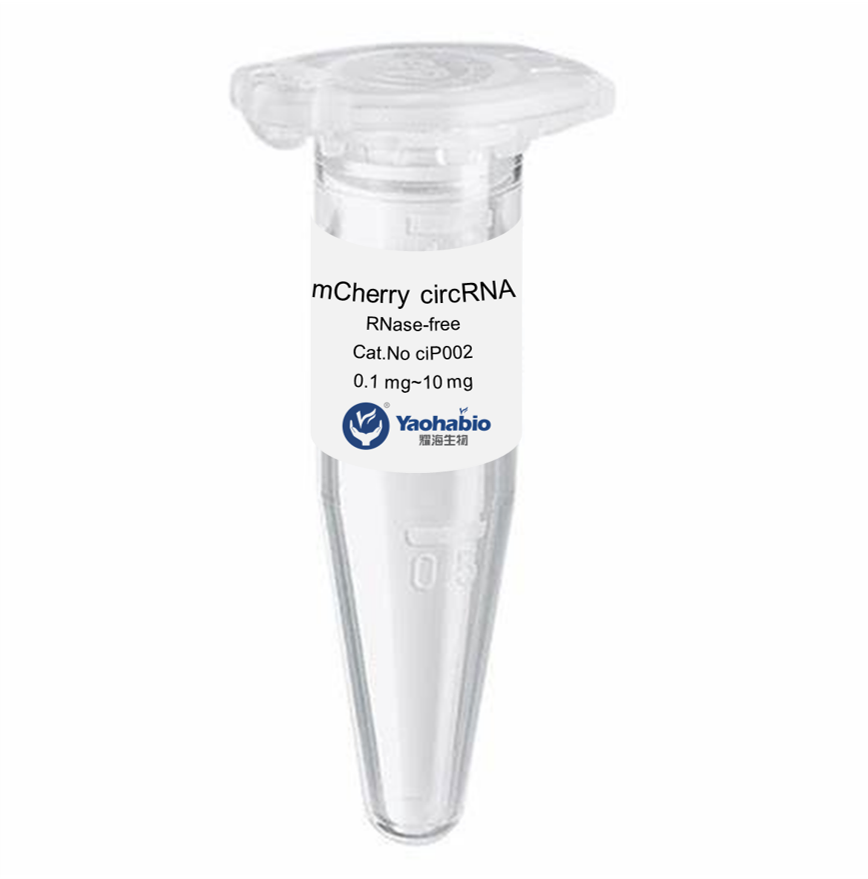
Kustomisasi, Efisiensi & Hemat Biaya
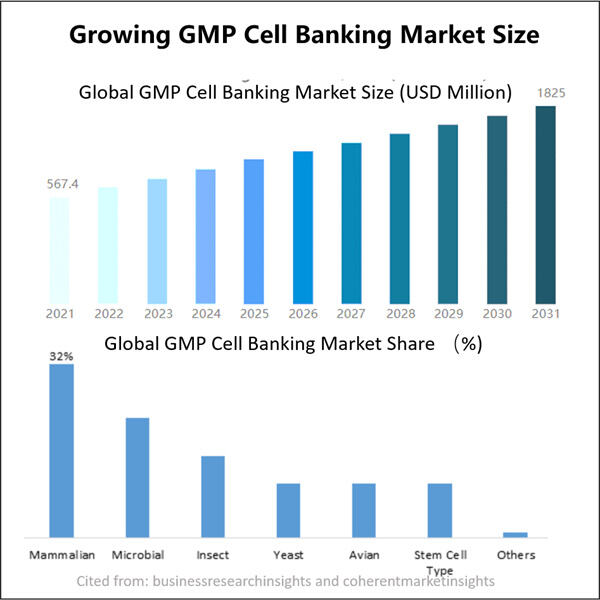
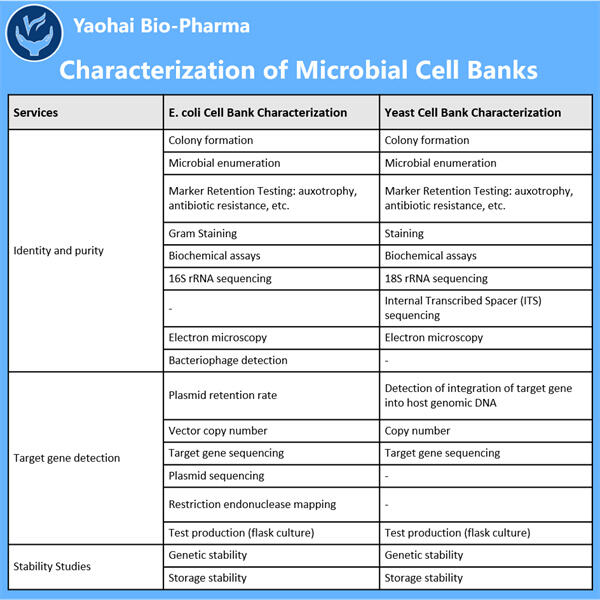
Yaohai Bio-Pharma adalah penyedia layanan GMP Microbial Cell Banking untuk biologis berbasis mikroba. Kami menawarkan solusi penelitian dan pengembangan khusus serta layanan manufaktur, sambil meminimalkan risiko. Kami telah terlibat dalam berbagai modali seperti vaksin subunit rekombinan, peptida hormon, sitokin faktor pertumbuhan, antibodi single-domain, enzim, plasmid DNA, mRNA, dan lainnya. Kami ahli dalam beberapa inang mikroba, seperti ragi ekstraseluler dan intraseluler (hasil hingga 15 gram per liter), sekresi periplasmik bakteri serta inklusi intraseluler larut (hasil hingga 10 gram/L). Selain itu, kami telah mengembangkan platform fermentasi mikroba BSL-2 untuk pengembangan vaksin bakteri. Kami memiliki catatan yang baik dalam meningkatkan proses produksi, sehingga meningkatkan hasil dan mengurangi biaya. Dengan tim teknologi yang sangat efisien, kami menjamin pengiriman proyek yang cepat dan andal serta membawa produk Anda lebih cepat ke pasar.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN