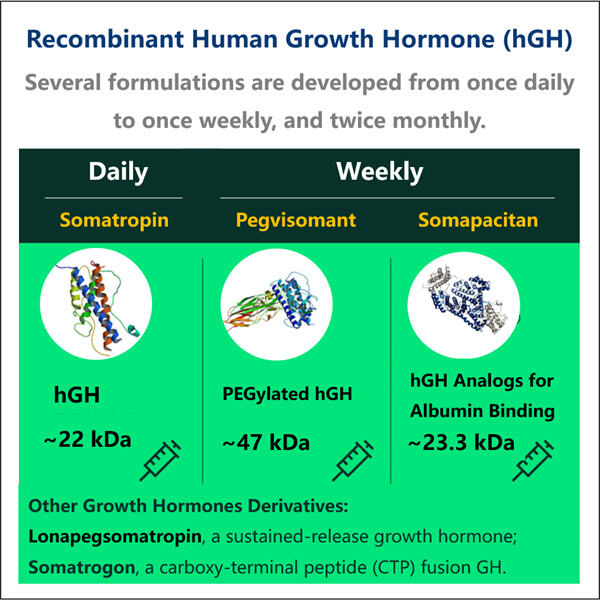
Banyak tahun yang lalu, ditemukan bahwa hormon pertumbuhan dapat membantu meningkatkan seberapa tinggi dan kuat beberapa orang yang tidak memproduksi cukup hormon tersebut secara alami. Hormon pertumbuhan sangat diperlukan bagi tubuh kita, tetapi tidak selalu bekerja sesuai keinginan kita. PARA ILMUWAN HARUS MELAKUKAN BEBERAPA HAL DAN MEMASTIKAN BAHWA HORMON PERTUMBUHAN TIDAK HANYA AMAN DIGUNAKAN OLEH MANUSIA, TETAPI JUGA BERFUNGSI DENGAN BAIK DALAM TUBUH MEREKA
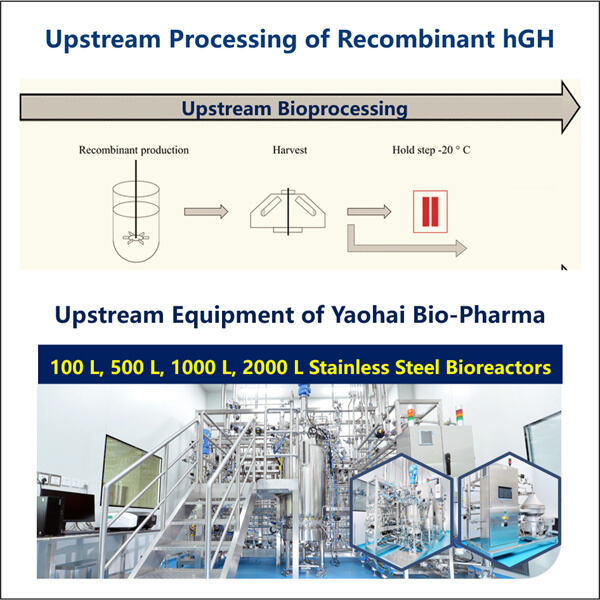
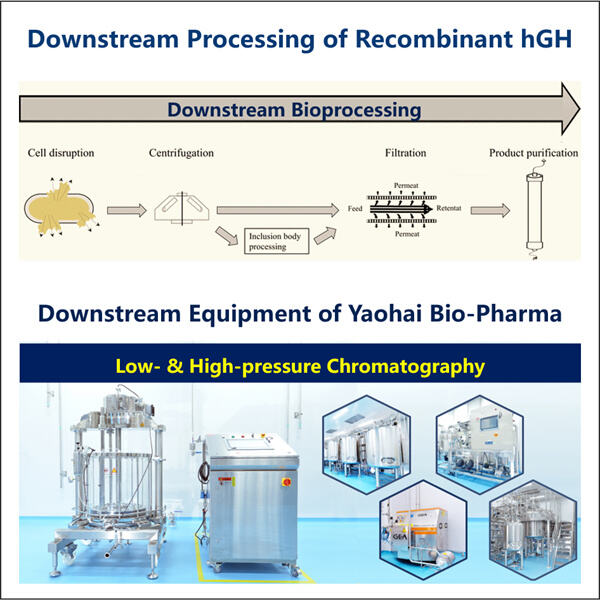
Hormon pertumbuhan dibuat, atau disintesis, dengan menggunakan Produksi Fragmen GLP-1 mesin yang dikenal sebagai bioreaktor. Mesin-mesin ini sebenarnya membuat hormon. Mereka melakukan hal ini dengan menyisipkan sebuah gen — sedikit materi genetik — ke dalam bakteri atau sel. Gen ini berfungsi sebagai panduan petunjuk. Hormon pertumbuhan, bukan protein biasa dari bakteri atau sel, itulah yang diperintahkan kepada sel untuk dibuat karena protein-protein tersebut sekarang tidak lagi diperlukan.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN