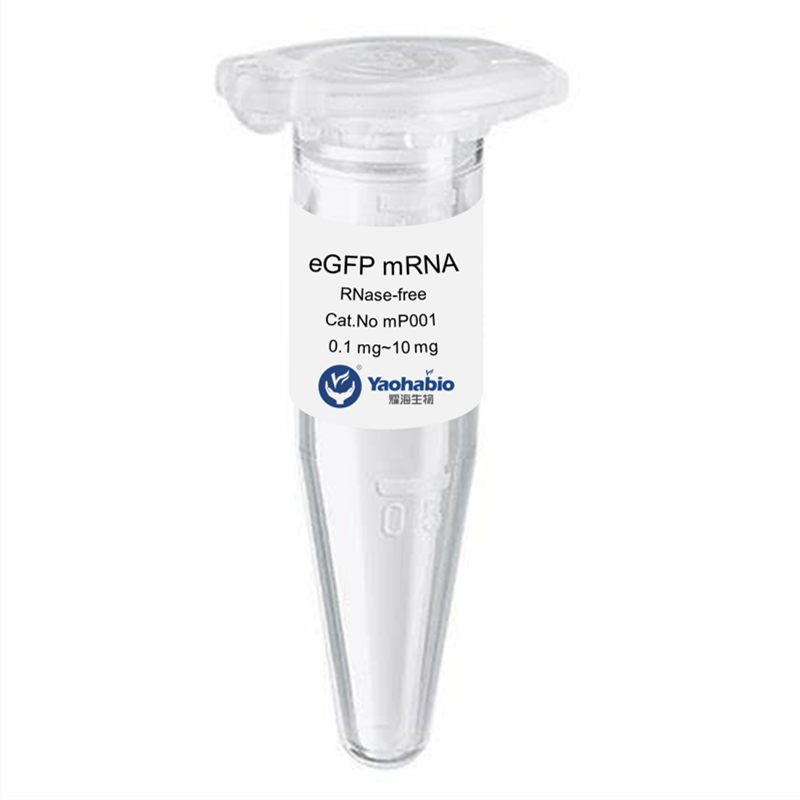
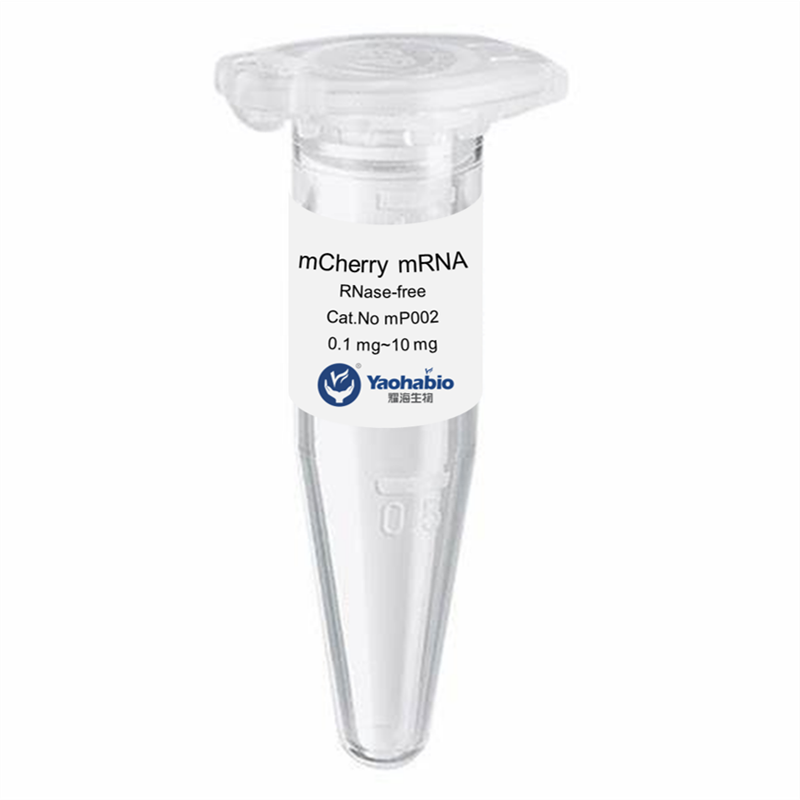
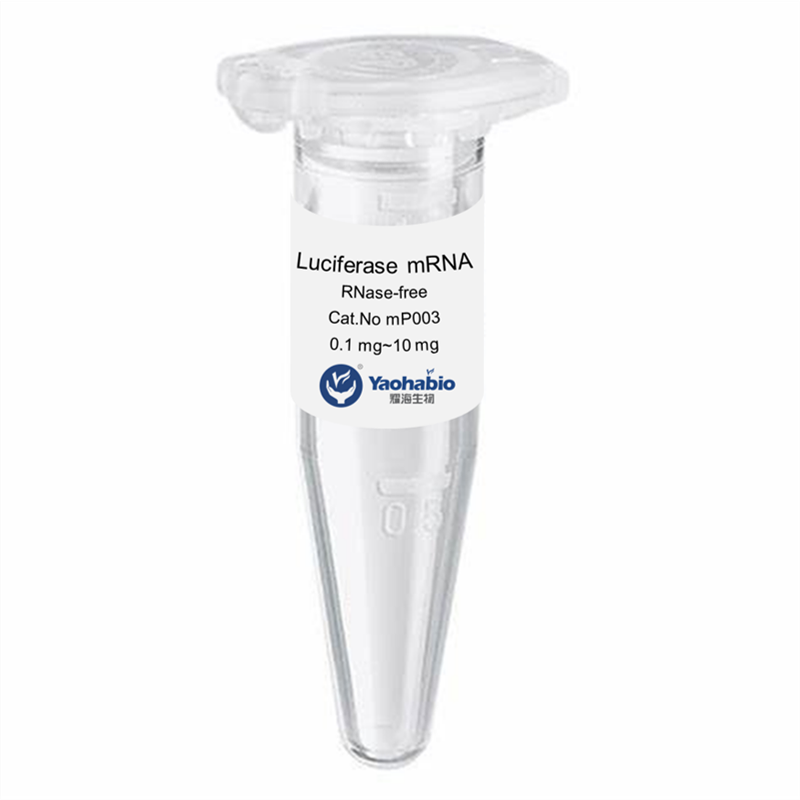
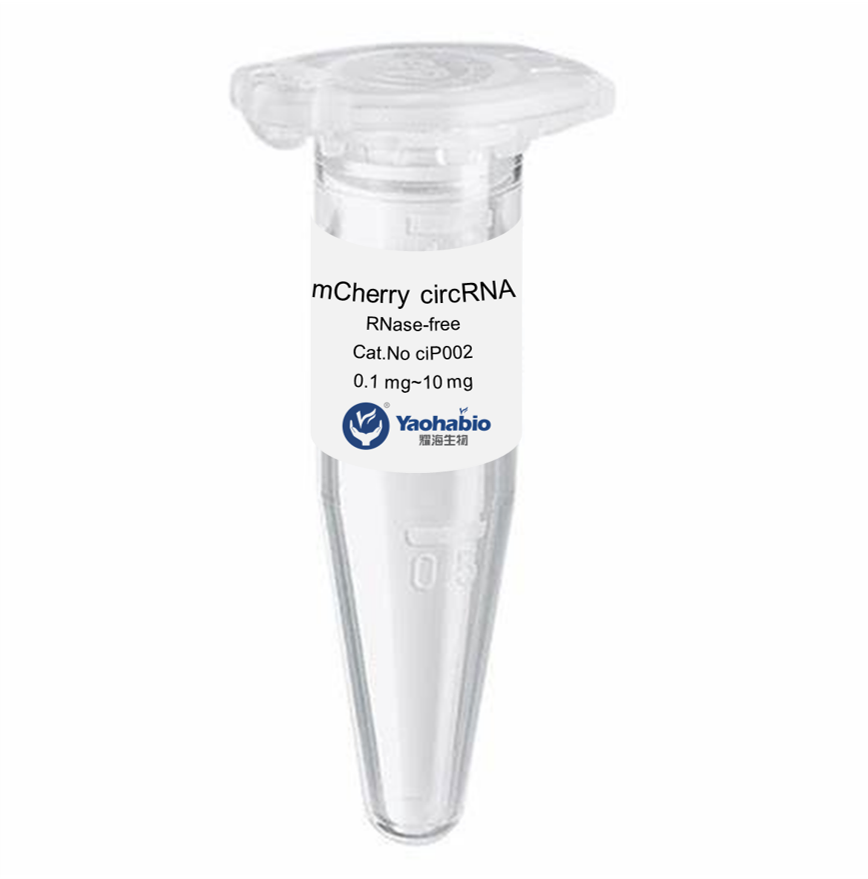
Kemampuan Profesional & Pengalaman Luas
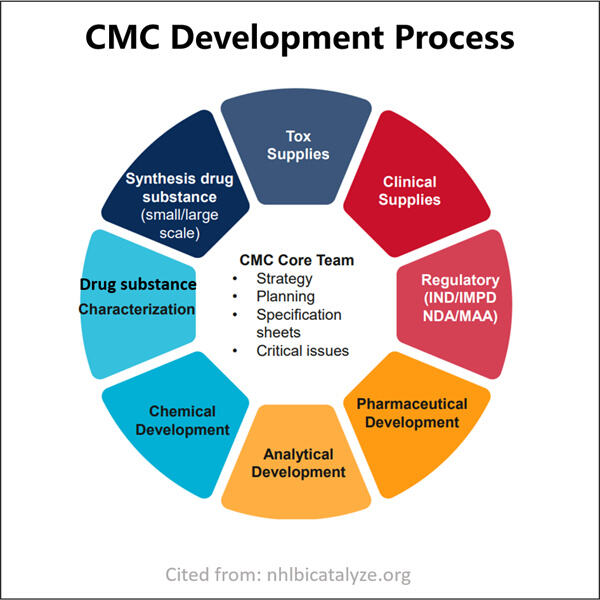
Yaohai Bio-Pharma adalah CDMO biologis mikroba terkemuka. Fokus utama kami adalah produksi bagian CMC dalam Pengajuan Obat dan terapi untuk mengobati hewan peliharaan, kesehatan manusia, dan kesehatan veteriner. Kami memiliki platform R&D dan teknologi manufaktur terdepan yang mencakup seluruh proses manufaktur, mulai dari pengembangan strain mikroba, perbankan sel, pengembangan proses dan metode, hingga produksi klinis dan komersial yang memastikan pengiriman solusi inovatif yang berhasil. Dengan berjalannya waktu, kami telah memperoleh pengetahuan mendalam tentang pemrosesan bio berbasis mikroba. Kami telah berhasil menyelesaikan lebih dari 200 proyek global dan membantu klien kami dalam menavigasi aturan dan regulasi dari US FDA, EU EMA, Australia TGA, dan China NMPA. Berkat pengalaman dan keahlian kami, kami mampu merespons dengan cepat terhadap permintaan pasar dan menyediakan layanan CDMO yang disesuaikan.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN