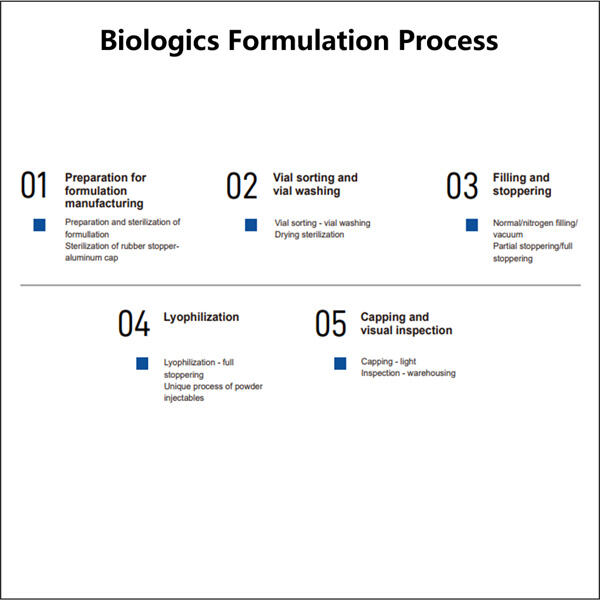
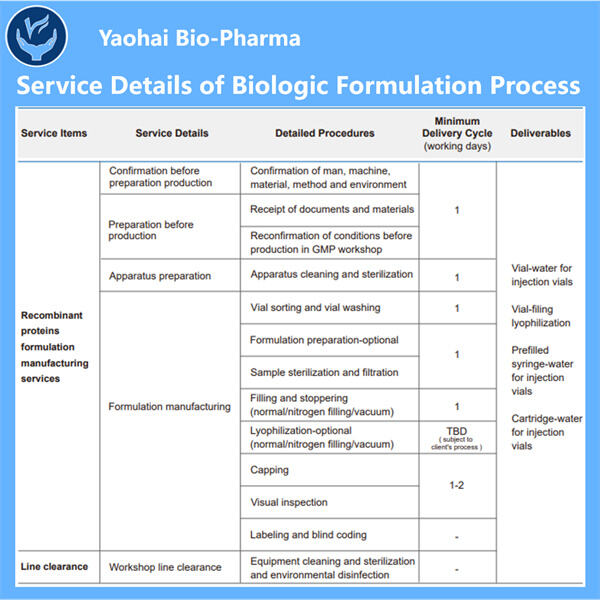
Biologics adalah obat-obatan unik yang bekerja sangat efektif melawan kondisi-kondisi yang sebelumnya kita anggap tidak dapat diobati. Mereka berasal dari organisme hidup, seperti tumbuhan dan hewan, dan memiliki kemampuan untuk menyerang jenis sel tertentu di tubuh kita untuk melawan penyakit. Ini termasuk kanker, diabetes, dan rematik. Namun, memproduksi obat-obatan ini bukanlah tugas yang mudah; mereka memiliki risiko tinggi terurai dan rentan terhadap ketidakstabilan jika penanganan tidak tepat. Itulah sebabnya para ilmuwan bekerja keras untuk mengembangkan biologics yang kuat dan aman yang dapat membantu pasien.
Produk biologis yang layak, aman, dan efektif tampak seperti tugas yang sangat sulit untuk dicapai memang. Mereka harus menguji berbagai macam bahan dan melihat apa yang bekerja dengan baik bersama-sama. Banyak faktor lain yang ikut bermain, seperti seberapa baik bahan-bahannya tercampur (kelarutan), suhu penyimpanan yang tepat, dan stabilitas produk akhir seiring waktu. Produk biologis dibuat sebagai seni dan ilmu pengetahuan, faktor-faktor ini harus seimbang dengan baik agar dapat bekerja secara efektif pada pasien di satu sisi, dan di sisi lain tidak merugikan pasien.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN