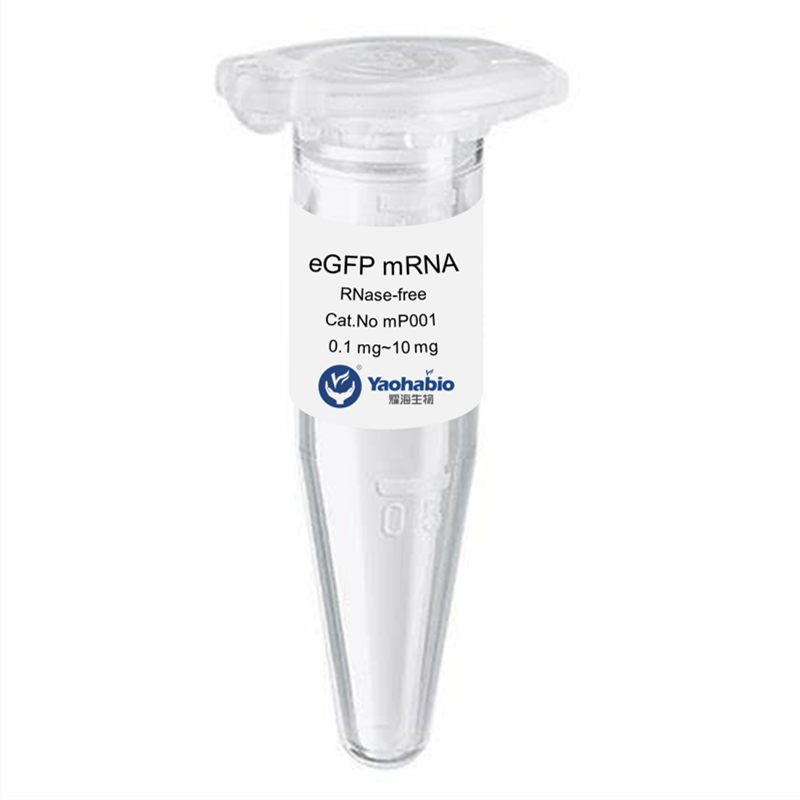
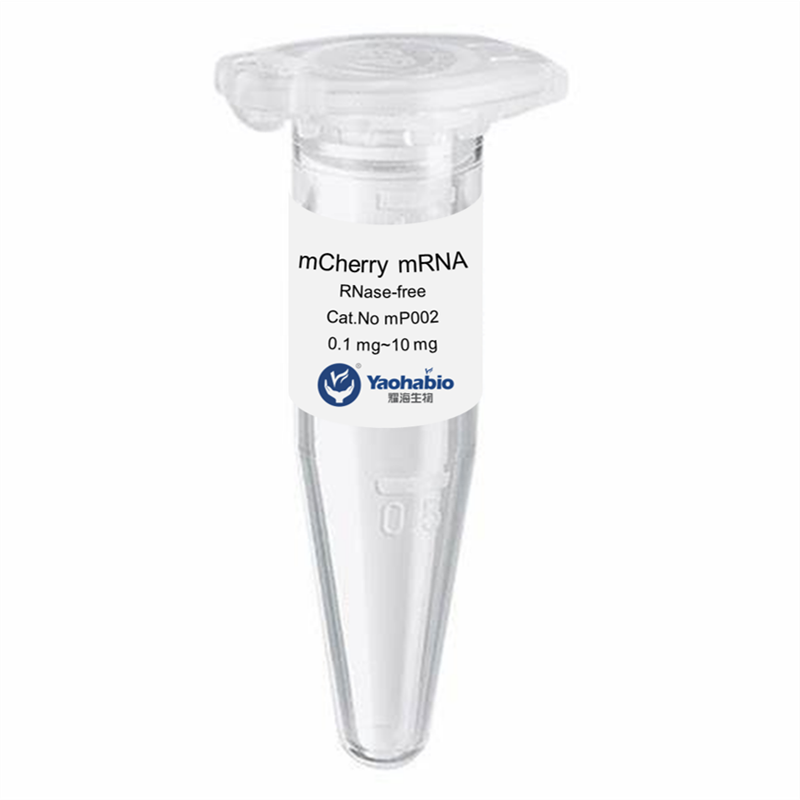
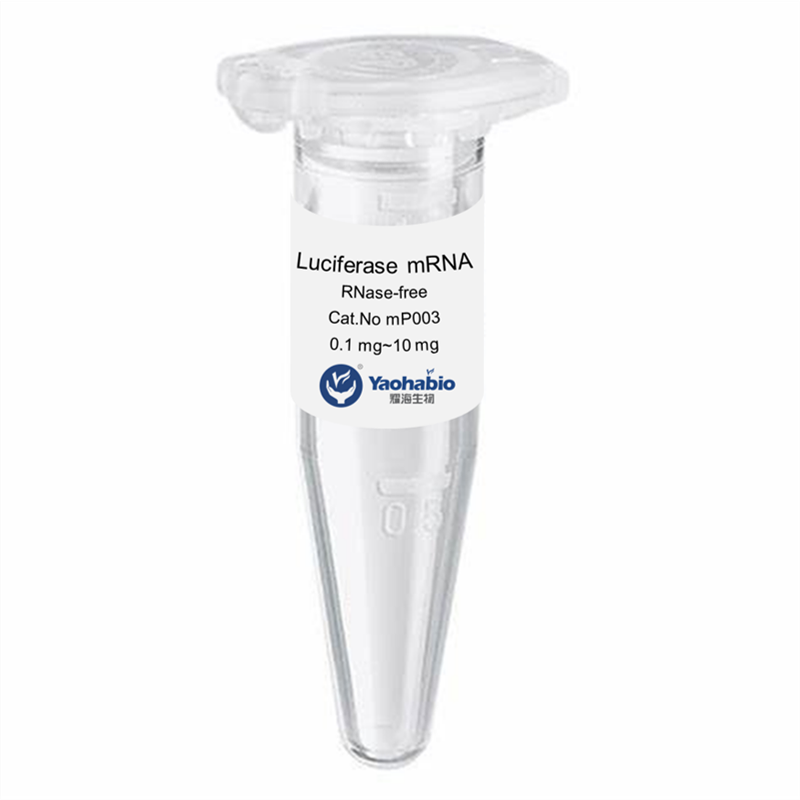
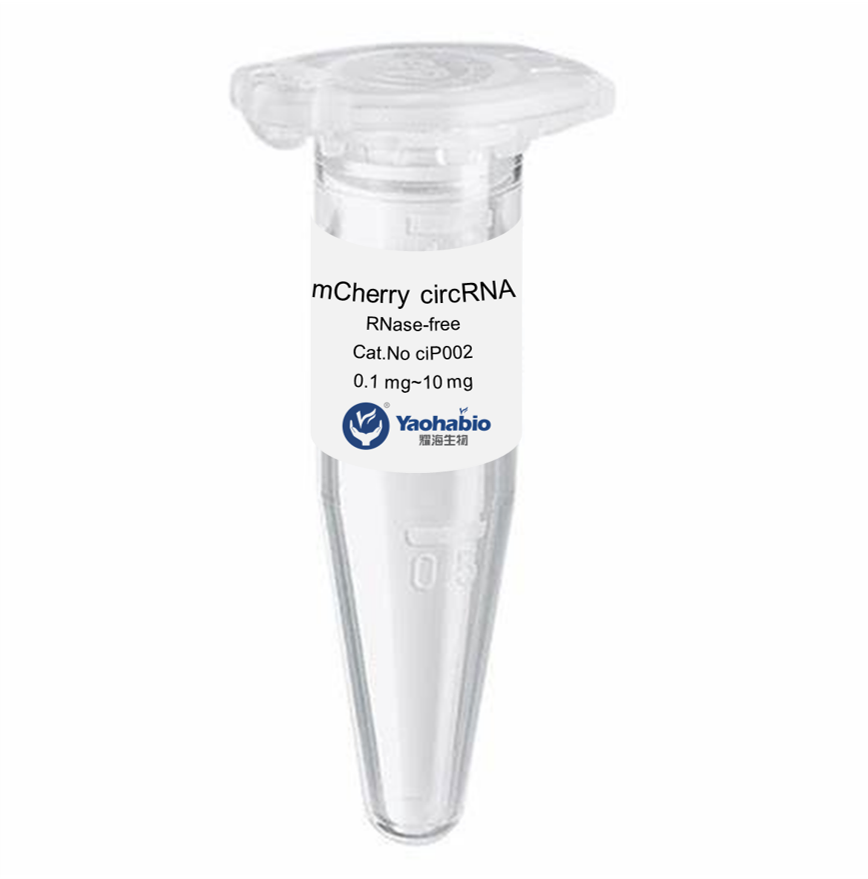
Personnalisation, Efficacité & Coût abordable
Yaohai Bio-Pharma a une grande expérience dans les produits biologiques dérivés de sources microbiennes. Nous fournissons des solutions d'RD sur mesure et une fabrication tout en minimisant les risques potentiels. Nous avons travaillé sur diverses modalités, y compris des vaccins à sous-unités recombinants, des peptides hormonaux, des cytokines et facteurs de croissance, des anticorps à domaine unique et enzymes, de l'ADN plasmidique et divers ARNm, et plus encore. Nous nous sommes spécialisés dans plusieurs micro-organismes, y compris le développement de processus de fragments ScFv, la sécrétion intracellulaire et extracellulaire (jusqu'à 15 g/L) et la bactérie soluble intracellulaire et corps d'inclusion (jusqu'à 10 g/L). Nous avons également mis en place une plateforme de fermentation BSL-2 pour créer des vaccins à base de bactéries. Nous sommes experts en amélioration des processus, en augmentation du rendement des produits et en réduction des coûts de production. Nous disposons d'une équipe technologique hautement efficace qui garantit une livraison de projets à temps et de haute qualité. Cela nous permet de faire parvenir vos produits uniques plus rapidement sur le marché.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN