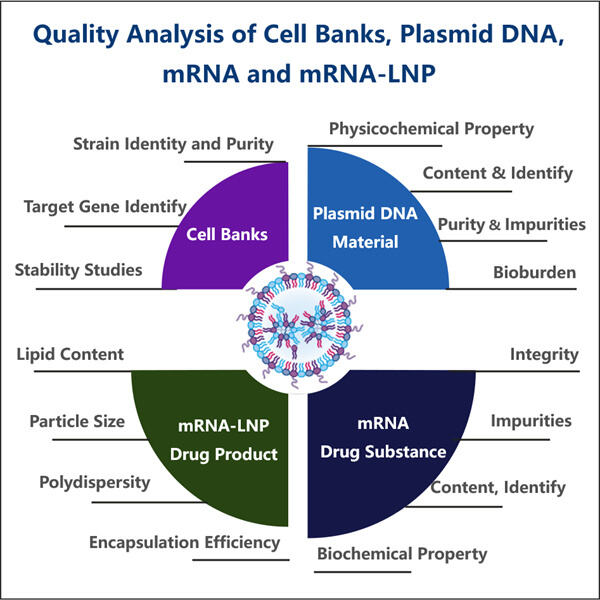
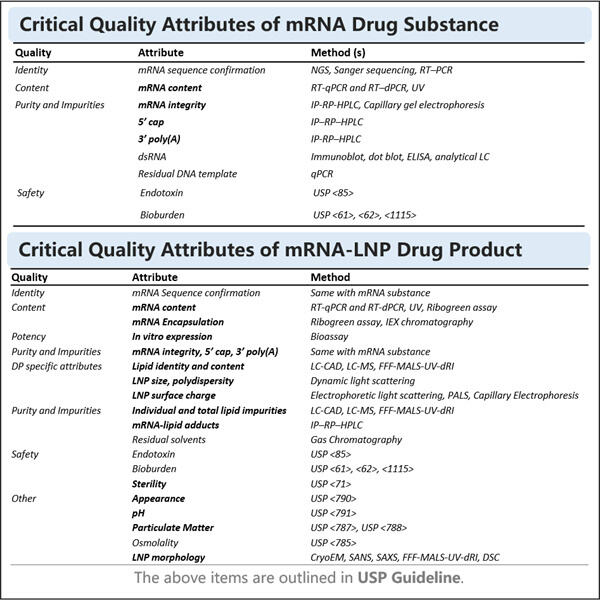
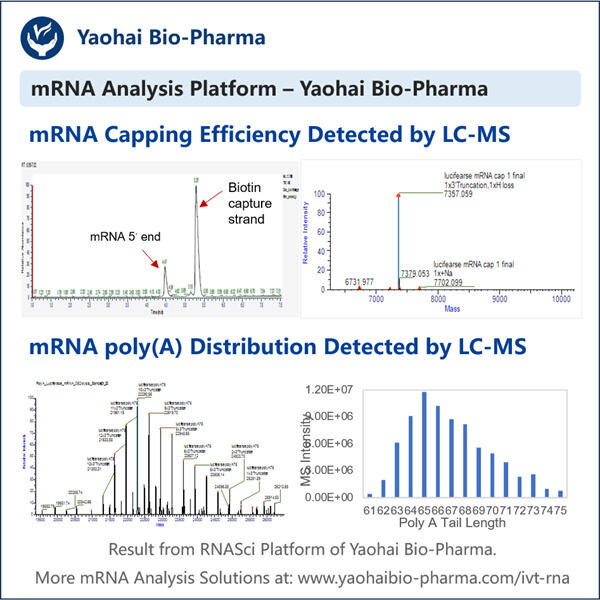
Anpassung, Effizienz & Kosteneffektivität
Yaohai Bio-Pharma hat Erfahrung in der Produktion von Biologika, die aus mRNA-Qualitätskontrolle erstellt wurden. Wir bieten maßgeschneiderte RD- und Fertigungslösungen, während wir sicherstellen, dass keine Risiken bestehen. Wir waren in verschiedenen Modalitäten wie Subeinheitsimpfstoffen rekombinant, Peptidhormonen, Cytokinen, Wachstumsfaktoren, Single-Domain-Antikörpern, Enzymen, Plasmid-DNA, MRNA und vielen mehr beteiligt. Wir sind Spezialisten für viele Mikroorganismen, einschließlich Hefextrazellulär und intrazellulärer Sekretion (Erträge bis zu 15g/L) sowie bakterieller intrazellulärer Löslichkeit und Inklusionskörper (Erträge bis zu 10g/L). Wir haben außerdem eine BSL-2-Fermentierungsplattform zur Erstellung bakterieller Impfstoffe entwickelt. Wir konzentrieren uns darauf, Prozesse zu verbessern, Produktzuwendungen zu erhöhen und Produktionskosten zu senken. Mit einem starken Technologie-Team können wir eine schnelle und verlässliche Projektrealisierung garantieren, die Ihr Produkt schneller auf den Markt bringt.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN