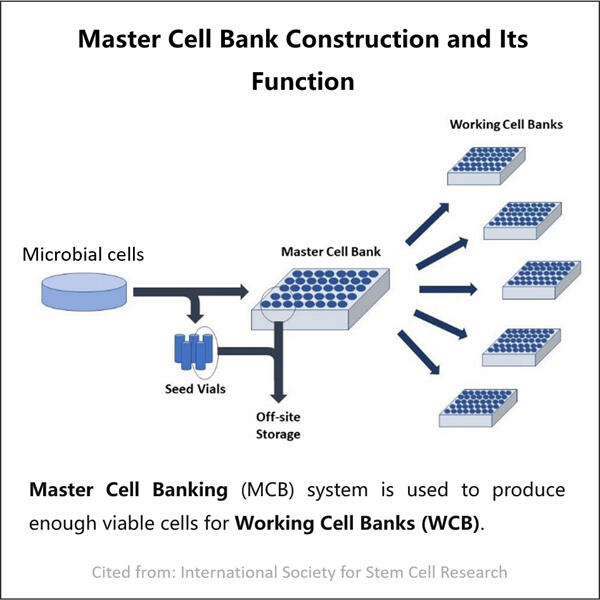
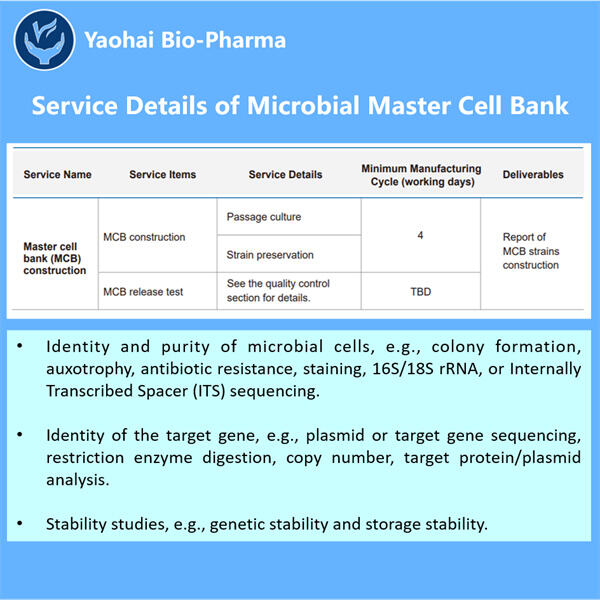
Ein Archiv von reinen und stabilen Mikroorganismen
Dies kann die Produktion von Biologika herausfordernder machen, denn wie ich bereits sagte, arbeiten wir mit Zellen – jedes Mal, wenn wir eine Charge von Biologika herstellen, könnte sie leicht unterschiedlich sein. Dies liegt daran, dass die Proteine, die den Menschen helfen sollen, aus jeder Charge von Zellen im Laufe der Zeit hergestellt werden und die Menge, die sie produzieren und liefern, variiert. Yaohai hat dann ein mikrobielles Masterzelldepot eingerichtet und die Lösung entwickelt. Diese Bank hatte eine Stärkung durch die Reinheit und Ehrlichkeit validierter natürlicher Chemikalien. Auf diese Weise können wir durch die Verwendung der gespeicherten Zellen Chargen herstellen, die in ihren Eigenschaften der ersten Charge ähnlich sind, und uns soergeben, wie konsistent unsere Medikamente sind. Daher ist es unerlässlich, dass wir jedes Medikament perfekt für die Patienten entwickeln.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN