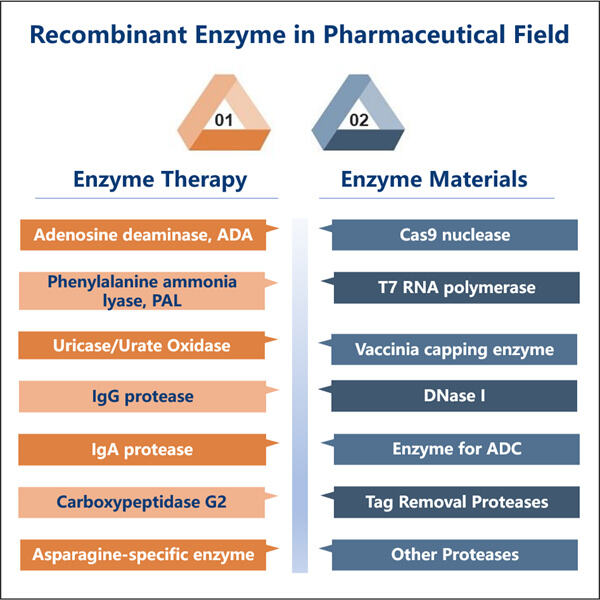
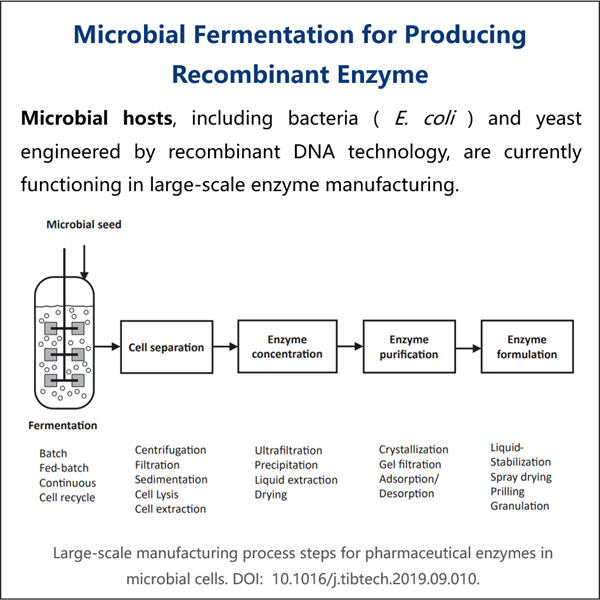
GMP = Good Manufacturing Practice. Es ist eine Reihe von Regeln, denen Unternehmen einhalten müssen, um sicherzustellen, dass ihre Produkte sicher und wirksam sind und Qualität bieten. Es ist ein bisschen wie ein Rezept, nur um sicherzustellen, dass nichts falsch gemacht wird. Dieses Gefühl können alle Unternehmen erleben und glauben, wenn sie sich an diese Regeln halten.
Rekombinant: Dies folgt auf rekombinant. Gen: Ein Begriff, der auf den Prozess verweist, bei dem neue DNA aus Teilen unterschiedlicher bestehender DNA erstellt wird. Stell dir DNA als Bauklötze vor, nutze zwei Blöcke, um etwas Neues und Spannendes zu erschaffen. Dies ist ein entscheidender Prozess in der Wissenschaft und hilft dabei, Enzyme zu schaffen, die sehr spezifische Funktionen in unserem Körper oder anderen Produkten ausführen können.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN