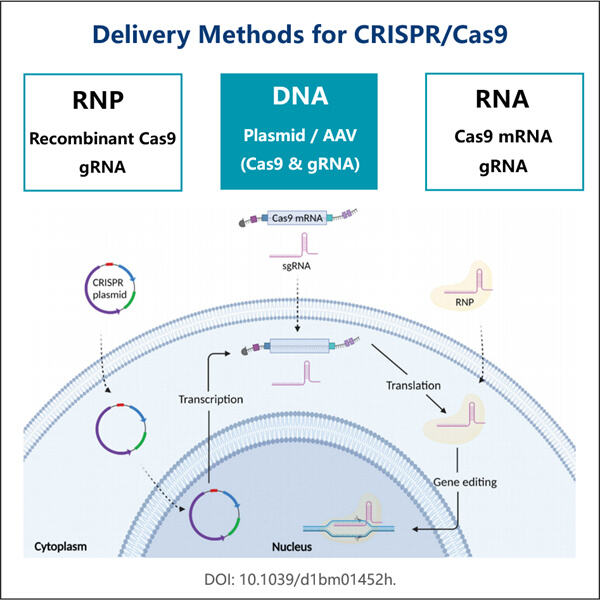
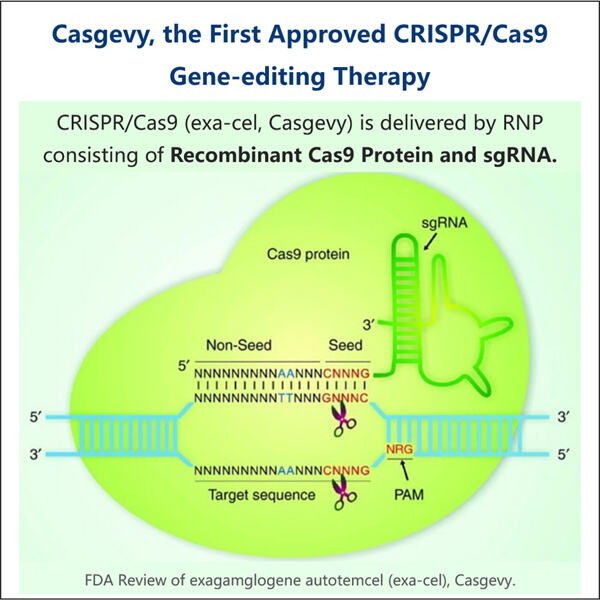
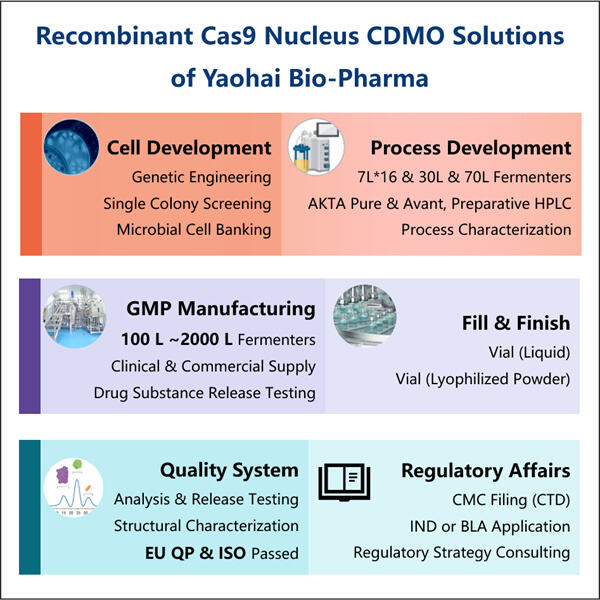
Die Herstellung von GMP Cas9 Nuclease ist eine Art, eine einzigartige Klasse von Enzymen namens Cas9 Nuclease zu erstellen. Diese Enzyme sind äußerst wichtig für die Technologie CRISPR, die Wissenschaftlern ermöglicht, Gene zu bearbeiten. GMP steht kurz für Good Manufacturing Practices. Wir verwenden diese Praktiken, um sicherzustellen, dass die Cas9 Nuclease-Enzyme, die wir produzieren, nicht nur sicher, sondern auch von hoher Qualität sind. Das zeigt, dass die Enzyme wie gewünscht funktionieren und für wissenschaftliche oder medizinische Zwecke verwendet werden können. Jeder Beitrag zum genetischen Editing hat noch einen langen Weg vor sich mit vielen weiteren Bedenken, aber die Herstellung von GMP Cas9 Nuclease könnte entscheidend sein. Mit allem gesagt, betrachten wir alle bei Yaohai diese Verantwortung mit höchster Wertschätzung. Wir achten sorgfältig darauf, Regeln und Maßnahmen einzuhalten, um sicherzustellen, dass wir konsequent die besten Qualitätsresins produzieren. Unser Qualitätskontrollteam führt eine detaillierte Überwachung durch jeden einzelnen Schritt im Streben nach der Herstellung dieser Enzyme. Dies DNA-Impfstoffherstellung ist es zu garantieren, dass die Enzyme gut gereinigt werden und wie erwartet richtig funktionieren. Und diese gründliche Prüfung ist der Weg, wie wir sicherstellen, dass unsere Enzyme in jeder Anwendung sicher und ordnungsgemäß eingesetzt werden.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN