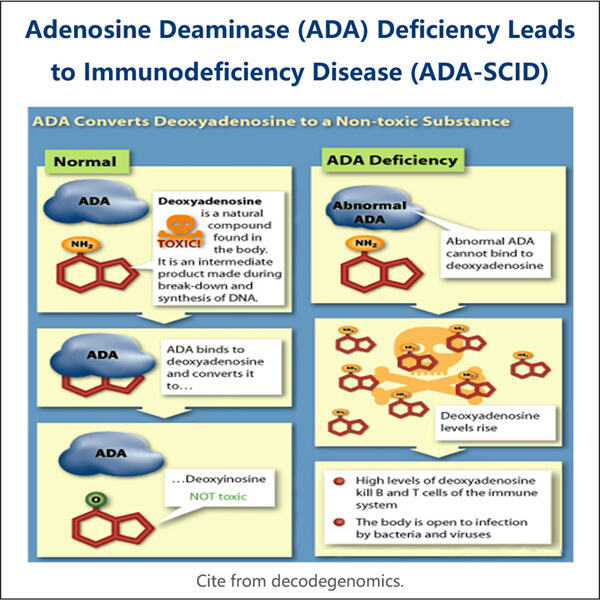
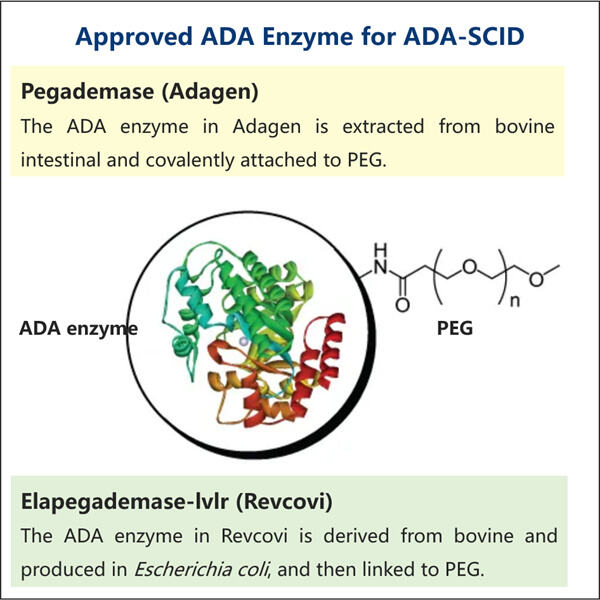
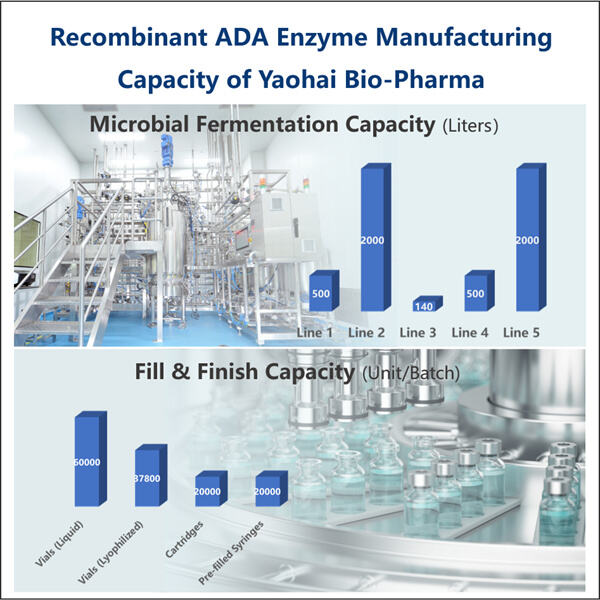
Der komplexe Prozess der ADA-Herstellung zu medizinischen Zwecken
Andererseits ist die Herstellung von Medikamenten auf Basis von ADA ein schwieriger und komplexer Prozess. Es ist ein langer, zeitaufwändiger Prozess, der viele Schritte zur Bestätigung für Wissenschaftler erfordert. Phase 1: Infusion von ADA-produzierenden speziellen Zellen. Wissenschaftler können diese Zellen durch Blut oder Knochenmark (ein weiches Gewebe innerhalb der Knochen) von einem menschlichen Spender sammeln. Wie bei der Sammlung, müssen Zellbiologen die Zellen in Containern wachsen lassen, wo ihr Wachstum überwacht werden kann. Dies ist ein wirklich sehr zeitaufwändiger Schritt und muss mit punktgenauer Perfektion ausgeführt werden, andernfalls werden die Zellen zu weich oder verlieren ihre Struktur.
Obwohl — wie auch bei der Entwicklung einer medizinischen ADA viele Vorschriften gelten, da die FDA so viele Regeln darüber hat, wann bestimmte Medikamente zu medizinischen Zwecken hergestellt werden dürfen. Dies geschieht, um sicherzustellen, dass die hergestellte ADA sauber, sicher und keine Probleme verursacht. Der erste Schritt dabei ist, dass wir die Gesundheit der Zellen überprüfen, bevor wir den Produktionsstadium für ADA erreichen und diese Zellen keine deformierten Fremdeinwanderer (oder Keime) beherbergen. Nach der Entwicklung muss die ADA auf ihre Sicherheit getestet werden. Daher ist das Testen zeitaufwendig und muss mehrfach überprüft werden, um die Qualität zu gewährleisten. Aber das gilt nur für Medikamente, die für medizinische Behandlungen verwendet werden und daher notwendig sind, wenn ein Medikament als wirksame Medikation nach den von Gesundheitsbehörden weltweit gesetzten Standards anerkannt werden soll und die vorgegebenen Regeln auch streng für ADA gelten.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN