CRDMO Modalities
Microbial CRDMO Solutions with excellence in quality and global regulatory compliance
Vaccine
Vaccines stimulate protective immunity through controlled exposure to antigens or whole pathogens. Traditional types include subunit, conjugate, toxoid, inactivated, and live-attenuated vaccines, while new technologies such as mRNA, DNA, and VLP vaccines are reshaping vaccine development.
Yaohai Bio-Pharma offers end-to-end microbial CRDMO services for vaccine projects, including DNA vaccines, mRNA vaccines, VLP vaccines, and more — enabling smooth transition from R&D to commercialization.
CRDMO Modalities
Service Platform
Service Highlights
CRDMO Modalities
Yaohai Bio-Pharma offers a range of vaccine CRDMO services utilizing microbial fermentation systems based on E. coli and yeast. 
Microbial Expression Systems

1. Genetic Vaccines
mRNA Vaccine
Based on messenger RNA encoding antigens to stimulate an immune response.
DNA Vaccine
Uses plasmid DNA to produce antigenic proteins in vivo.
2. Protein/Peptide-based Vaccines
Subunit Vaccine
Uses specific protein fragments of pathogens.
Protein/Peptide-based Therapeutic Vaccine
Targets specific diseases (e.g., cancer) with designed peptides/proteins.
Virus-like Particle (VLP) Vaccine
Mimics the structure of viruses without genetic material.
3. Polysaccharide & Conjugate Vaccines
Polysaccharide Vaccine
Contains purified bacterial polysaccharides.
Conjugate Vaccine
Combines polysaccharides with proteins to enhance immune response.
4. Toxoid Vaccines
Toxoid Vaccine
Uses inactivated bacterial toxins to elicit immunity.
5. Microbial-based Vaccines
Live Attenuated Vaccine
Uses weakened live microbes to induce a strong immune response.
Inactivated Vaccine
Uses killed microbes; safer but may require boosters.
Microbial-vector Vaccine
Uses engineered microbe-like bacteria or viruses to deliver genetic material.
Viral Vector Vaccine
Uses modified viruses (e.g., adenovirus) as delivery vehicles.
Service Platform
Yaohai Bio-Pharma offers end-to-end CRDMO services for vaccine development, including microbial cell banking, process development, analytical method development, GMP manufacturing, quality control, and regulatory filing support — enabling efficient transition from early-stage development to commercial production.
Microbial CRDMO Service Platform for Vaccine
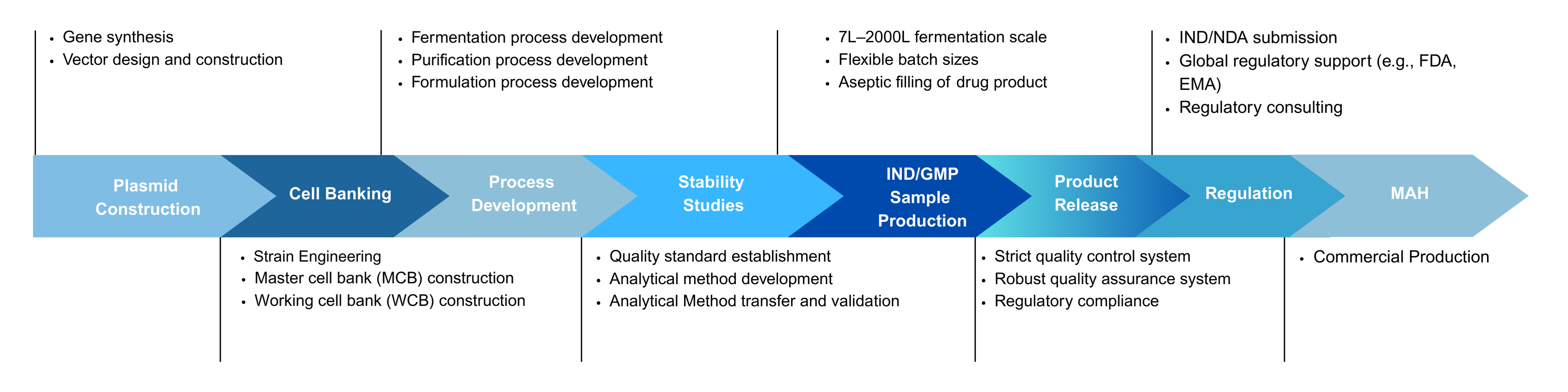
Yaohai Bio-Pharma Offers One-Stop Vaccine CRDMO Solutions
- Microbial Strain Development and Screening
- Microbial Cell Banking (PCB/MCB/WCB)
- Upstream Process Development
- Downstream Process Development
- Formulation Development
- GMP Manufacturing
- Fill and Finish
- Analytical and Testing
- Regulatory Affairs*
Service Highlights
Lifecycle Management for Vaccine Projects
- One-stop service: early R&D → clinical manufacturing → commercial-scale GMP production
- Integrated planning for process validation, stability, and regulatory filing
- Tailored CRDMO solutions to accelerate vaccine innovation
Robust Vaccine CRDMO Technology Platform
- Seamless tech transfer with GMP workflows for early-stage vaccine projects
- Supporting diverse vaccine modalities: mRNA, DNA, protein subunit, VLP, live attenuated, inactivated vaccines
Core Vaccine Expertise
- More than 10 years of experience in microbial-based vaccine development and biomanufacturing
- Dedicated teams for formulation, QC, and scalable production
- Familiar with global vaccine regulatory requirements and technical review criteria
- Proactive risk assessment ensures accelerated timelines and cost control
Regulatory & Compliance Excellence
- In-depth understanding of vaccine-specific IND/NDA submission pathways
- Real-time monitoring of global regulatory updates and policy shifts
- Comprehensive regulatory documentation templates for efficient compliance
Related products
Let's start talking with us
Our value
Serve With Heart
Build Future Together
Yaohai Bio-Pharma delivers tailored solutions for global clients, meeting diverse project requirements with excellence and efficiency.
Proven Project Excellence
Proven track record of over 100 successful projects, spanning preclinical studies and Phase I/II/III clinical trials, including US–China dual submissions and Australian regulatory filings.
Regulatory Compliance Assurance
A robust GMP-aligned quality system with standardized SOPs, fully compliant with global regulatory guidelines across the entire product lifecycle.
Expert Team Support
Powered by industry-leading scientists and cross-functional experts, our team delivers CRDMO projects with speed, precision, and deep technical insight.