CRDMO Modalities
Microbial CRDMO Solutions with excellence in quality and global regulatory compliance
Recombinant Protein
Recombinant proteins are produced using recombinant DNA technology, primarily expressed in host cells such as E. coli or yeast. They are widely used in therapeutic protein drugs, vaccine development, drug delivery, immunoassays, and diagnostic reagents. Recombinant proteins also play key roles in antibody target screening, CAR-T cell therapy, ADCs, enzymes, viral proteins, and cytokine research.
Yaohai Bio-Pharma provides one-stop CMC research and GMP manufacturing services for a wide range of recombinant proteins, including peptides, cytokines, growth factors, enzymes, and virus-like particles (VLPs). With a well-established CRDMO platform and extensive project experience, the company is capable of meeting diverse commercial needs across different stages of recombinant protein drug development.
CRDMO Modalities
Service Platform
Service Highlights
CRDMO Modalities
Yaohai Bio-Pharma leverages an integrated CMC development and cGMP manufacturing platform to produce high-quality recombinant proteins using E. coli and yeast expression systems.
Microbial Expression Systems

Recombinant Protein CRDMO Modalities:
- Recombinant Growth Factors
- Virus-Like Particles (VLPs)
- Recombinant Cytokines
- Recombinant Enzymes
- Recombinant GLP-1
- Recombinant Insulins
- Growth Hormones
- Parathyroid Hormone (PTH)
- Leptin
- Glucagon
- Thrombopoietin (TPO)
Service Platform
With a mature microbial CRDMO platform specializing in E. coli and yeast, we provide comprehensive services including microbial cell banking, process development, IND and NDA support, as well as GMP manufacturing and global regulatory support — fully equipped to support drug development and commercialization needs.
Microbial CRDMO Service Platform for Recombinant Protein
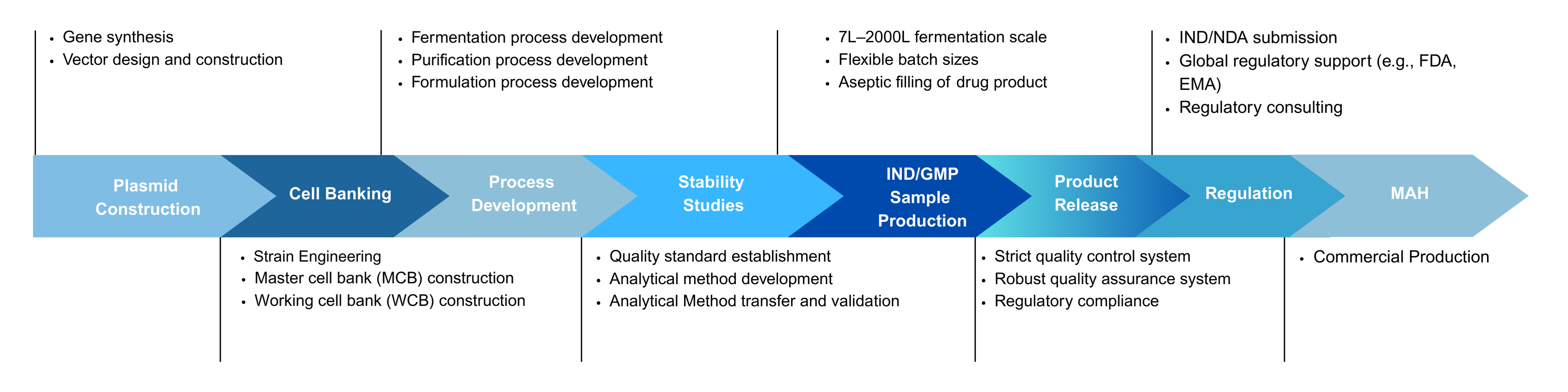
Yaohai Bio-Pharma Offers One-Stop Recombinant Protein CRDMO Solutions
- Microbial Cell Banking (PCB/MCB/WCB)
- Upstream Process Development
- Downstream Process Development
- Formulation Development
- GMP Manufacturing
- Fill and Finish
- Analytical and Testing
- Regulatory Affairs
Service Highlights
Integrated Recombinant Protein Process Development Capabilities
- Diverse protein development experience: Covering recombinant peptides, cytokines, carrier proteins, enzymes, allergens, VLPs, vaccines, etc
- Tag-free purification platform: Mature platform reducing process-related impurities
- Defines Critical Quality Attributes (CQA) & Critical Process Parameters (CPP) via Design of Experiments (DoE)
Proven Project Experience
- 100+ CMC projects for recombinant proteins
- 99% project success delivery rate
- Mature process development technology to reduce process development time
Comprehensive Manufacturing Capabilities
- Scalable production: 50L – 2,000L
- 2 drug substance production lines: Liquid & lyophilized vials, pre-filled syringe/cartridge
- High-purity expression
Quality Management System
- Full compliance with the global Pharmacopoeia & GMP Standards
- Lifecycle quality control (stability studies under ICH guidelines)
- Strict risk management framework
End-to-End CRDMO Solutions
- From early R&D to commercial production
- Support all clinical phases (I/II/III) & MAH
- Integrated project management
Efficient Empowerment
- Dedicated CRDMO team
- Cost-efficient innovation to save costs
- Accelerated seamless tech transfer
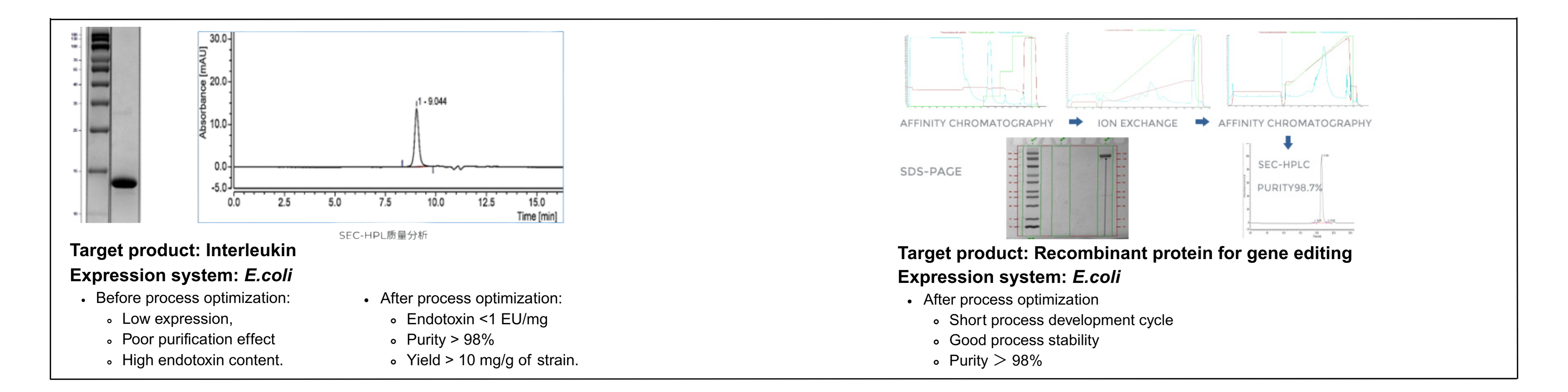
Recombinant Protein CRDMO Service Case
Related products
Let's start talking with us
Our value
Serve With Heart
Build Future Together
Yaohai Bio-Pharma delivers tailored solutions for global clients, meeting diverse project requirements with excellence and efficiency.
Proven Project Excellence
Proven track record of over 100 successful projects, spanning preclinical studies and Phase I/II/III clinical trials, including US–China dual submissions and Australian regulatory filings.
Regulatory Compliance Assurance
A robust GMP-aligned quality system with standardized SOPs, fully compliant with global regulatory guidelines across the entire product lifecycle.
Expert Team Support
Powered by industry-leading scientists and cross-functional experts, our team delivers CRDMO projects with speed, precision, and deep technical insight.