CRDMO Modalities
Microbial CRDMO Solutions with excellence in quality and global regulatory compliance
Plasmid DNA
Plasmid DNA is a circular, self-replicating molecule widely used as a vector in gene therapy and vaccine production. It serves as a raw material for viral vector construction (LV, AAV), a transcription template for mRNA vaccines, or the final product for DNA vaccines.
Yaohai Bio-Pharma has established GMP-compliant platforms for circular and linear plasmid production, offering plasmid manufacturing services spanning from preclinical and clinical stages to commercial production. This enables cell and gene therapy innovators to accelerate R&D and commercialization with high-efficiency support.
CRDMO Modalities
Service Platform
Service Highlights
CRDMO Modalities
Yaohai Bio-Pharma delivers end-to-end microbial CRDMO services for plasmid DNA, enabling innovative therapies across critical fields:
- Viral Gene Therapy (AAV vectors)
- Cell Therapy Applications
- DNA Vaccines & Therapeutics
- mRNA Production Support
Plasmid DNA CRDMO Project Experience
Service Platform
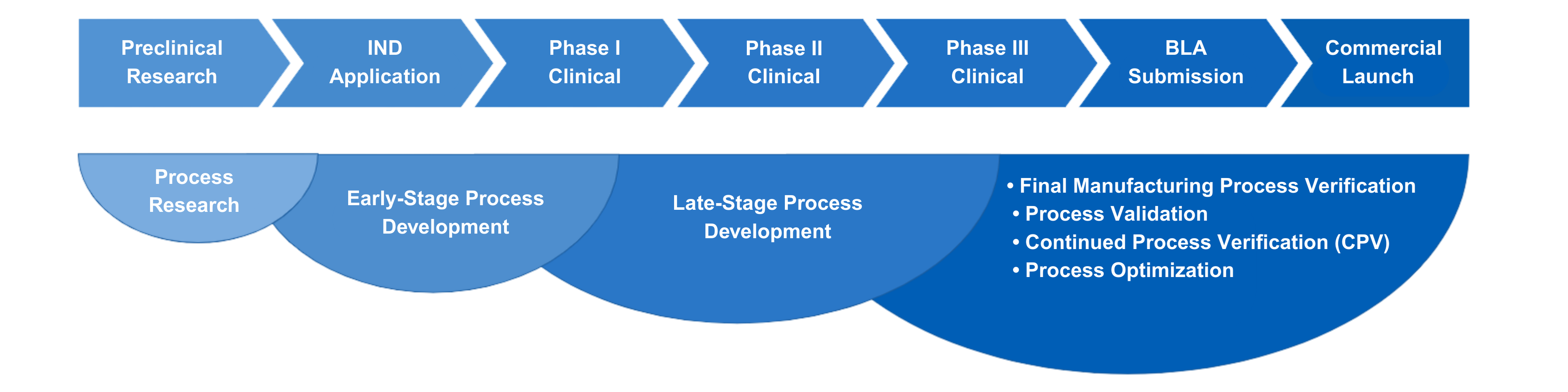
Yaohai Bio-Pharma offers end-to-end plasmid DNA CRDMO services from vector design to GMP production, supporting gene and cell therapy programs from preclinical to commercialization. Our GMP-compliant platform enables the production of supercoiled and linearized plasmids, with integrated services that cover construction, process development, quality testing, and regulatory support. 
Microbial CRDMO Service Platform for Plasmid DNA
Service Content:
| Plasmid Construction | Strain preservation & stability studies | Analytical method development & validation |
| Microbial Cell Line Development | Upstream process development | Gram-scale plasmid production & testing (GMP-like) |
| Three-Tier Microbial Cell Banking (PCB/MCB/WCB) | Formulation process development | GMP plasmid production & release |
| Cell bank characterization & passage stability studies | Process validation | Regulatory filing support |
Service Highlights
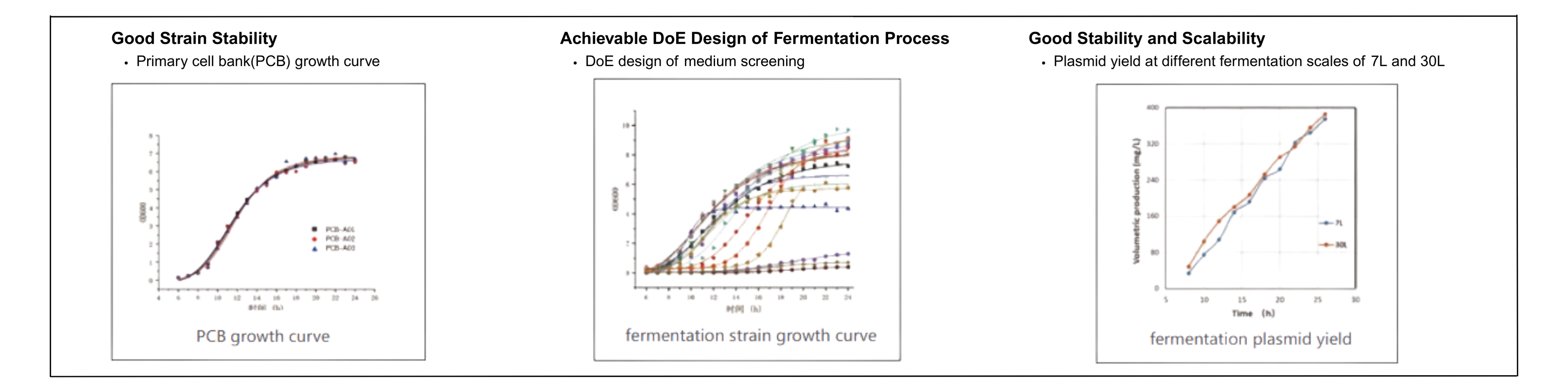
High-quality Strain
- Clear strain traceability; stable strains with high expression efficiency
Robust Plasmid Manufacturing Platform
- 7L to 2000L fermentation scale to meet diverse production needs
Rich Project Experience
- Extensive CMC and clinical GMP experience across various plasmid applications; proven track record in IND clinical submissions.
Plasmid Process Development
- QbD-driven process development using DoE to define CQAs and CPPs, ensuring consistent quality and robust processes
- Antibiotic-free, animal-origin-free fermentation process
- High-density fermentation and high expression up to 600 mg/L
- HCD residuals <2 µg/mg; Purity >98%
Plasmid DNA CRDMO Service Case
Related products
Let's start talking with us
Our value
Serve With Heart
Build Future Together
Yaohai Bio-Pharma delivers tailored solutions for global clients, meeting diverse project requirements with excellence and efficiency.
Proven Project Excellence
Proven track record of over 100 successful projects, spanning preclinical studies and Phase I/II/III clinical trials, including US–China dual submissions and Australian regulatory filings.
Regulatory Compliance Assurance
A robust GMP-aligned quality system with standardized SOPs, fully compliant with global regulatory guidelines across the entire product lifecycle.
Expert Team Support
Powered by industry-leading scientists and cross-functional experts, our team delivers CRDMO projects with speed, precision, and deep technical insight.