Recombinant plasmids are an important vector in the field of cell and gene therapy (CGT), which can be used as,
-
DNA Therapeutics (Naked plasmid DNA for therapy) - Naked plasmid as a gene expression vector, as an alternative to protein/enzyme replacement therapy.
-
DNA Vaccines for prophylactic and therapeutic use - Plasmid as gene vector, which expresses antigens from virus, bacteria, or cancer cell.
- Starting materials for viral vector production - Recombinant plasmids may be used to produce lentivirus (LV) and adeno-associated virus (AAV) for virus vector vaccine, gene therapy or gene editing.
- Starting materials for mRNA/circRNA production - Linearized plasmid, as templates for in vitro transcription, are key materials for mRNA/circRNA vaccines or drugs.
1 Naked Plasmid DNA
1.1 Naked Plasmid DNA for Human Use
The gene therapy drugs currently on the market primarily use viral vectors such as AAV and LLV. However, research has reported that angiogenic factors gene therapy mediated by viral or cellular vectors may lead to the formation of vascular tumors in mouse hearts. To avoid prolonged expression of angiogenic factors, the use of naked plasmids with plasmid DNA as the gene therapy vector express lower level of target protein in vivo and is considered a preferable choice.
Therefore, the primary development focus of naked plasmid therapeutics is angiogenic factors gene therapy. As of now, there are a total of two approved naked plasmid drugs for human use globally: Neovasculgen, launched in Russia in 2011, and Collategene, introduced to the Japanese market in 2019. Several other naked plasmid drugs are currently in the Phase II-III clinical stages. Encoding genes include HGF, VEGF-A, SDF-1 (CXCL12), and among others.
1.2 Naked Plasmid DNA for Animal Use
Different form human medicines, DNA vaccines have been more successful for animal use, including veterinary and pet.
Table 1. Licensed DNA Therapeutics for Human and Animal Use
|
Application
|
Product
|
Species
|
Target
|
Indication
|
Company
|
Licensed Date/ Country
|
|
Gene Therapy
|
Neovasculgen, Cambiogenplasmid, PI-VEGF165
|
Human
|
VEGF-A
|
CLI, critical limb ischemia
|
Human Stem Cell Institute
|
2011/ Russia
|
|
Gene Therapy
|
Collategene, beperminogene perplasmid, AMG0001
|
Human
|
HGF
|
CLI, critical limb ischemia
|
AnGes
|
2019/Japan
|
|
Gene Therapy
|
LifeTideSW5
|
Swine
|
Porcine growth hormone releasing hormone (GHRH)
|
Increase the number of piglets weaned.
|
VGX Animal Health
|
2008/Australia
|
|
Cancer Immunotherapy
|
Oncept
|
Dogs
|
Tyrosinase
|
Oral malignant melanoma (OMM)
|
Merial, Boehringer Ingelheim Animal Health
|
2010/USA
|
|
Antimicrobials
|
Zelnate
|
Bovine
|
Pending Update
|
Bovine respiratory disease (BRD) due to Mannheimia haemolytica
|
Diamond Animal Health, Bayer
|
2013/USA
|
2 DNA vaccine
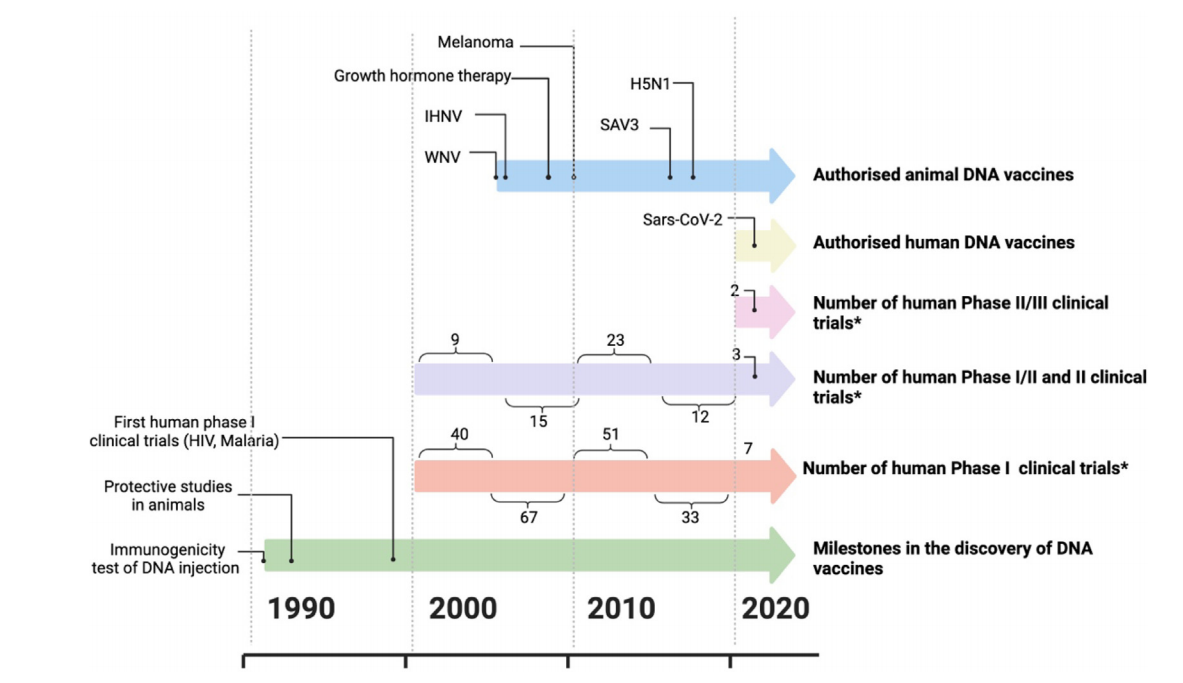
Fig 1. Development of DNA Vaccines
2.1 DNA vaccine for human use
The low immunogenicity in humans still poses a big challenge to DNA vaccine application in spite of the progress in animal models.
Furthermore, the exploration of DNA vaccines for infectious diseases, such as HIV, tuberculosis, and malaria, has prompted the development of diverse optimization strategies in subsequent years.
Table 2. Licensed DNA Vaccines for Human Use
|
Uses
|
Brand Name
|
Target/Indication
|
Stage
|
Company
|
|
Prophylactic vaccine
|
ZyCoV-D
|
Spike-protein; SARS-CoV-2
|
Emergency Use Authorization in India
|
Zydus Cadila
|
2.2 DNA vaccine for animal use
DNA vaccines in veterinary applications have made great progress as various products have obtained licenses for infectious diseases, such as cancer immunotherapy and gene therapy applications.
Table 3. Licensed DNA Vaccines for Animal Use
|
Uses
|
Brand Name
|
Species
|
Target/Indication
|
Company
|
Licensed Date/ Country
|
|
Prophylactic vaccine
|
West Nile-Innovator
|
Horses
|
West Nile Virus (WNV)
|
USA CDC, Fort Dodge Animal Health
|
2005/USA
|
|
Apex-IHN
|
Salmon
|
Infectious haematopoietic necrosis virus (IHNV)
|
Novartis Animal Health
|
2005/Canada
|
|
Clynav
|
Salmon
|
Salmon alphavirus subtype 3 (SAV3)
|
Elanco Animal Health
|
2016/EU
|
|
ExactVac
|
Poultry
|
Avian Influenza A (H5N1)
|
AgriLabs
|
2017/USA
|
3 Plasmid DNA as Materials for mRNA or Virus Vector Production
mRNA and circular mRNA (circRNA) have been extensively employed in vaccine development research. Linearized plasmid DNA serves as the requisite transcription template for IVT mRNA, facilitated by T7 RNA polymerase.
The viral vector stands out as the most efficient method for gene transfer, enabling the targeted modification of specific cell types or tissues and allowing for manipulation to express therapeutic genes. In the production of viral vectors, plasmid DNA plays a crucial role.
Yaohai Bio-Pharma Offers One-Stop CDMO Solution for Plasmid DNA
Reference:
[1] Pagliari S, Dema B, Sanchez-Martinez A, Montalvo Zurbia-Flores G, Rollier CS. DNA Vaccines: History, Molecular Mechanisms and Future Perspectives. J Mol Biol. 2023 Dec 1;435(23):168297. doi: 10.1016/j.jmb.2023.168297.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN


