
ABOUT US
Dedicated CRDMO solutions provider specializing in microbial expression systems
Who we are
Global Leader in Microbial CRDMO
Yaohai Bio-Pharma, founded in 2010, is a CRDMO specializing in microbial expression systems. We focus on the development and manufacturing of recombinant proteins, plasmids, nano-bodies, vaccines, mRNA, and circRNA, providing integrated CRO/CDMO/MAH services across the entire biologics lifecycle.
- Expert Team
- Scalable Biomanufacturing
- Rich Project Experience
- Regulatory Compliance
Information
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

0
+
Years Experience
Expert in Microbial CRDMO
Shaping the Future of Biomanufacturing
Yaohai Bio-Pharma is a customer-centric company specializing in diverse biologics manufacturing and process development,
and it provides GMP-compliant, high-quality microbial CRDMO solutions.
and it provides GMP-compliant, high-quality microbial CRDMO solutions.
0
+
Global Clients
0
+
Delivered Projects
0
+m²
GMP Manufacturing Area
0
+
Successful Audits
Company Milestones
2025 & Future

Advancing to become a global leader in microbial CRDMO
2024

Obtained ISO certifications and EU QP qualification
2022

Began developing an integrated global CRDMO platform
2021

Expanded fermentation lines to 7,500 L total capacity
Added a pre-filled syringe DP line with an annual output of 10 million units
2020

Series B funding exceeded RMB 100 million
Launched new production line
2019
Series A funding of RMB 51 million
Expanded fermentation scale to 2,000L
2018

Signed first MAH service agreement
Initiated new production line
Scaled manufacturing capacity
2016
Entered microbial CDMO sector and began strategic focus
2012

Commissioned an 8,000 m² GMP manufacturing facility
Obtained drug manufacturing license
2010

Company founded in August, 2010; established in China Medical City, Taizhou

BUSINESS ETHICS
Quality
Collaboration
Customer-centric
We are committed to complying with global regulations and upholding the highest standards of business ethics. We also protect clients’ intellectual property and data security, foster an ethical corporate culture, and enhance employee well-being.
Mission
Shaping Global Standards to Accelerate Drug Development and Advance Global Health.
Vision
To become a globally leading and sustainable partner in microbial CRDMO, driving long-term value for the biopharmaceutical industry.
Safeguarding Data
Protecting Trust
We enforce strict confidentiality and data protection standards across all operations, ensuring every service safeguards our clients’ intellectual property — the core of our shared trust.

WANT TO LEARN MORE?
Discover Our Expertise
Explore our tailored CRDMO services and innovative solutions designed to support your biologics development
from concept to commercialization.
from concept to commercialization.
CRDMO Modalities
We provide one-stop CRDMO solutions for biologics, with a strong focus on recombinant proteins, plasmids, nano-bodies, and novel recombinant vaccines.
CRDMO Solutions
We provide comprehensive CRDMO services—from CMC process development to MAH-based commercial production—enabling the full journey from DNA to commercialization.
News&Article
Access the latest updates on company news, events, and technical articles.
FAQ
Frequently Ask Questions
Ut fames nibh euismod fermentum justo velit ultricies nunc duis placerat taciti. Torquent quam nisi integer fermentum accumsan litora. Efficitur potenti hac imperdiet laoreet vel vivamus placerat platea augue taciti. Dictum ultricies efficitur metus aliquet consectetuer pulvinar vivamus eget.
Ut fames nibh euismod fermentum justo velit ultricies nunc duis placerat taciti. Torquent quam nisi integer fermentum accumsan litora. Efficitur potenti hac imperdiet laoreet vel vivamus placerat platea augue taciti. Dictum ultricies efficitur metus aliquet consectetuer pulvinar vivamus eget.
Ut fames nibh euismod fermentum justo velit ultricies nunc duis placerat taciti. Torquent quam nisi integer fermentum accumsan litora. Efficitur potenti hac imperdiet laoreet vel vivamus placerat platea augue taciti. Dictum ultricies efficitur metus aliquet consectetuer pulvinar vivamus eget.
Ut fames nibh euismod fermentum justo velit ultricies nunc duis placerat taciti. Torquent quam nisi integer fermentum accumsan litora. Efficitur potenti hac imperdiet laoreet vel vivamus placerat platea augue taciti. Dictum ultricies efficitur metus aliquet consectetuer pulvinar vivamus eget.
Ut fames nibh euismod fermentum justo velit ultricies nunc duis placerat taciti. Torquent quam nisi integer fermentum accumsan litora. Efficitur potenti hac imperdiet laoreet vel vivamus placerat platea augue taciti. Dictum ultricies efficitur metus aliquet consectetuer pulvinar vivamus eget.
Driving Environmental Sustainability Through Responsible Operations
We prioritize energy efficiency use throughout all aspects of our operations. By implementing practical measures, minimizing waste, promoting recycling, and reducing emissions, we actively work to lower our environmental footprint and protect the planet.

Related Article


The Overview of DS Manufacturing Expert
December 25, 2025
No Comments
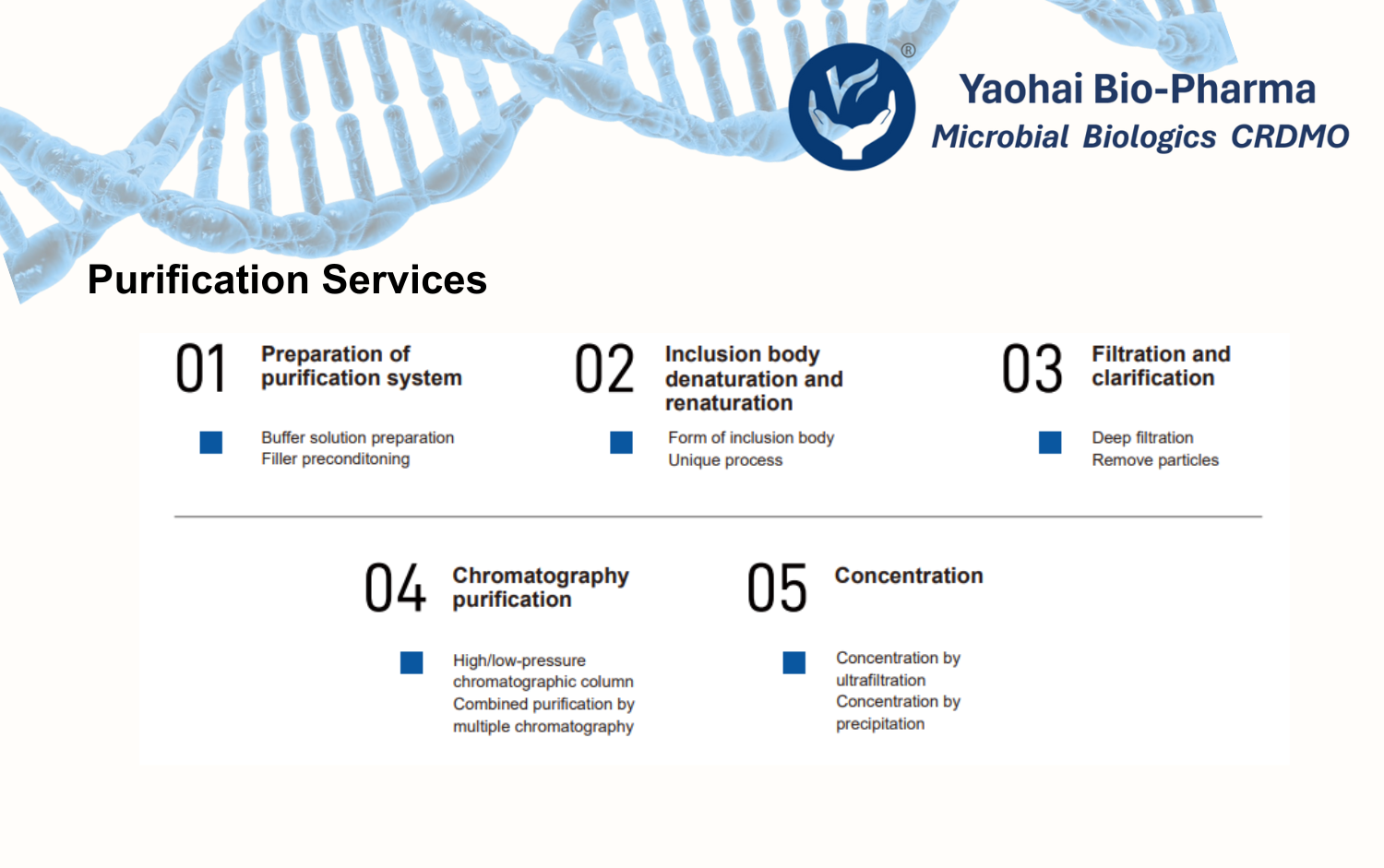
Yaohai Purification is the Best Standard
November 28, 2025
No Comments
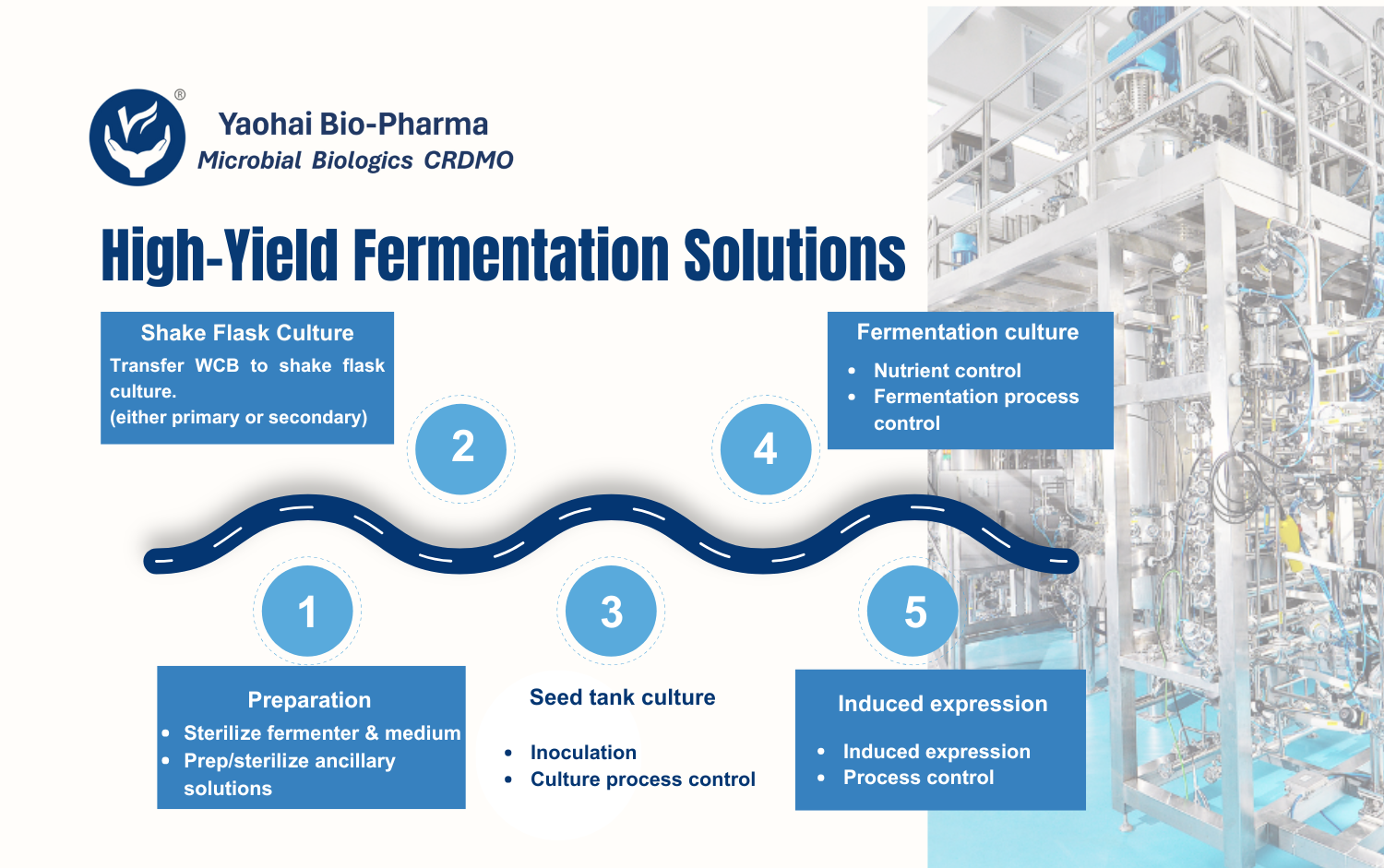
Microbial Fermentation is the New Trend
November 10, 2025
No Comments
Let's Accelerate Your Project Success




