Biologics development faces growing challenges. These include high R&D costs, complex technologies, and long clinical timelines. Companies must balance limited resources with tight schedules. At the same time, global regulatory requirements continue to evolve, adding pressure on quality and compliance.
Choosing the right CDMO partner is therefore critical. The right partner can reduce risk, shorten timelines, and ensure consistent product quality throughout development and manufacturing.
With extensive project experience, mature technology platforms, and a robust quality system, Yaohai Bio-Pharma provides integrated CDMO solutions for biologics developers worldwide. To date, we have successfully delivered over 200 projects, supporting programs from early research to IND submission, GMP manufacturing, and commercial manufacturing.
Integrated Drug Substance CDMO Platform
Yaohai Bio-Pharma offers one-stop drug substance manufacturing services for preclinical, clinical, and commercial-stage products. Our GMP-compliant facilities are built on Quality by Design principles and meet the requirements of NMPA, FDA, and EMA.
We operate five independent drug substance production lines and support a wide range of fermentation scales. This flexible setup allows us to match different development stages and process needs with speed and efficiency.
Manufacturing Scope and Capabilities
- Modality types supported include recombinant proteins, nucleic acid products, and nano-bodies.
- Microbial Expression systems cover E. coli, Pichia pastoris, and other microbial platforms.
- Development stages range from IND-enabling production to Phase I–III clinical supply and commercial manufacturing.
Our teams adapt quickly to different process requirements and deliver GMP manufacturing with strong execution and control.
High-Capacity, Flexible GMP Manufacturing
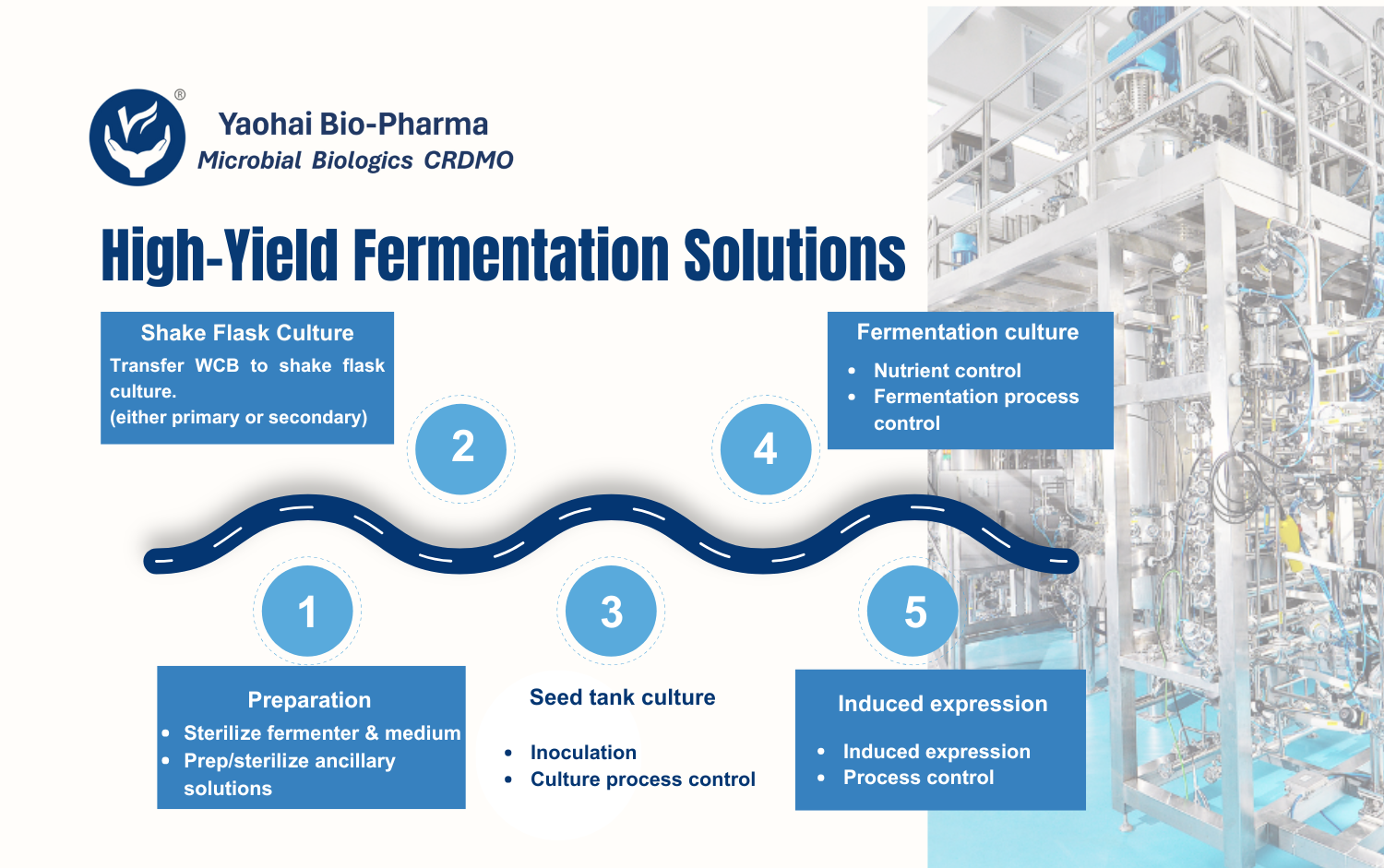
Yaohai Bio-Pharma operates more than 10,000 square meters of GMP-qualified drug substance manufacturing space. Multiple fermentation scales are available, including 50–100 L, 200 L, 500 L, 1,000 L, and 2,000 L, allowing smooth scale-up across development stages.
Upstream and downstream operations are housed in independent areas and equipped with internationally recognized manufacturing systems.
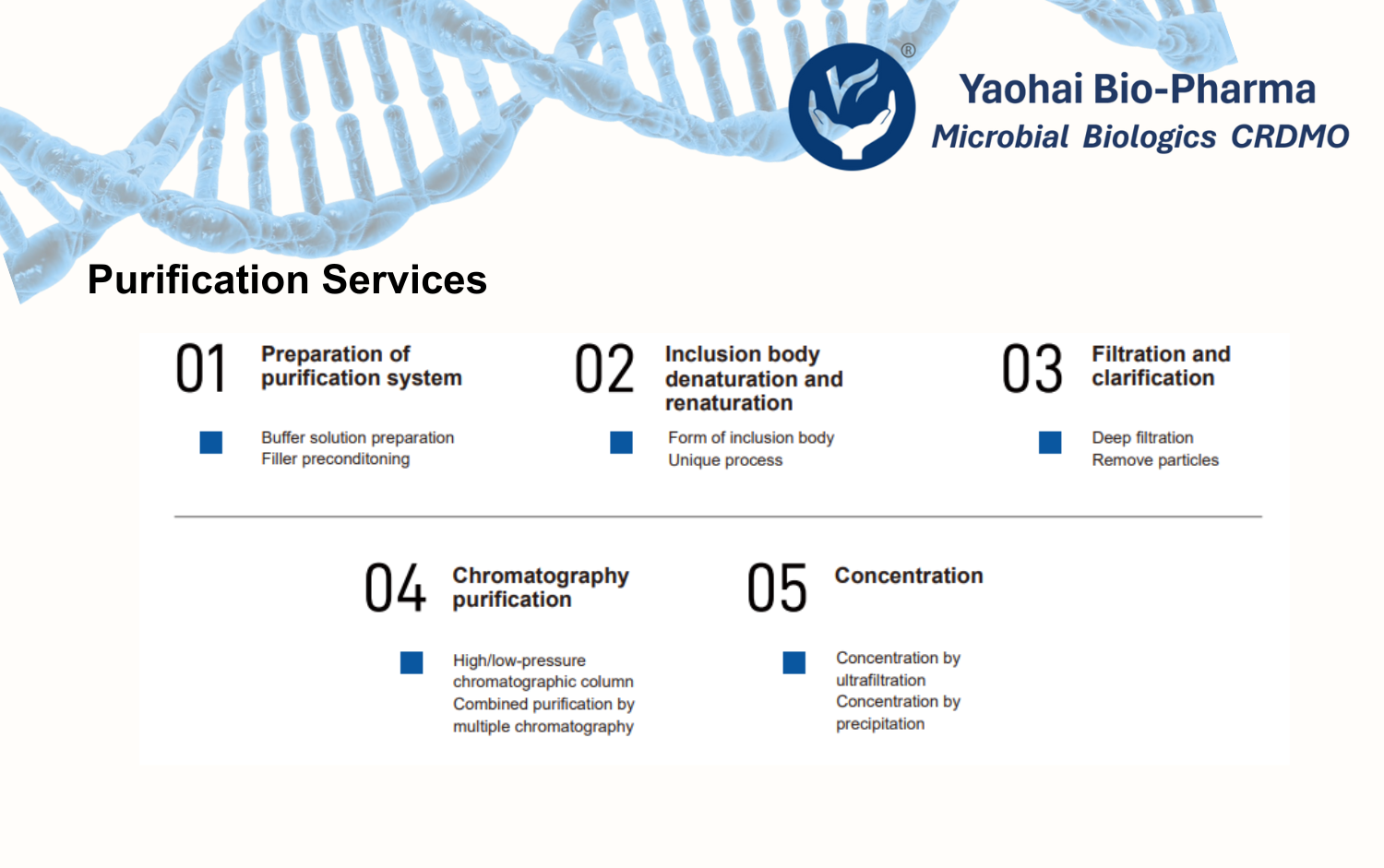
Upstream manufacturing includes five production lines with a total capacity of 7,500 L, supported by a full range of fermenters. Downstream processing is supported by five purification lines, equipped with low-, medium-, and high-pressure chromatography systems as well as ultrafiltration units, covering a broad process scale range.
Facility design follows GMP best practices. HVAC systems, water systems, and utilities have completed full IQ, OQ, and PQ validation. All equipment is qualified, and instrument calibration is performed comprehensively. PQ services can also be provided upon request.
Quality-Driven Execution
Our manufacturing operations are supported by experienced cross-functional teams. Dedicated QA, operations QA, and validation professionals ensure that quality systems are executed efficiently and consistently. From production to release, data integrity, traceability, and compliance are maintained throughout the entire process.
About Yaohai Bio-Pharma
Yaohai Bio-Pharma is a CRDMO with extensive expertise in microbial expression systems. We focus on recombinant proteins, nucleic acid drugs, nanobodies, plasmid DNA, and innovative vaccines. From R&D to commercial manufacturing, we offer full-lifecycle solutions. Our mission is to build an open, integrated CRO/CDMO/MAH platform that supports global partners from discovery to market.