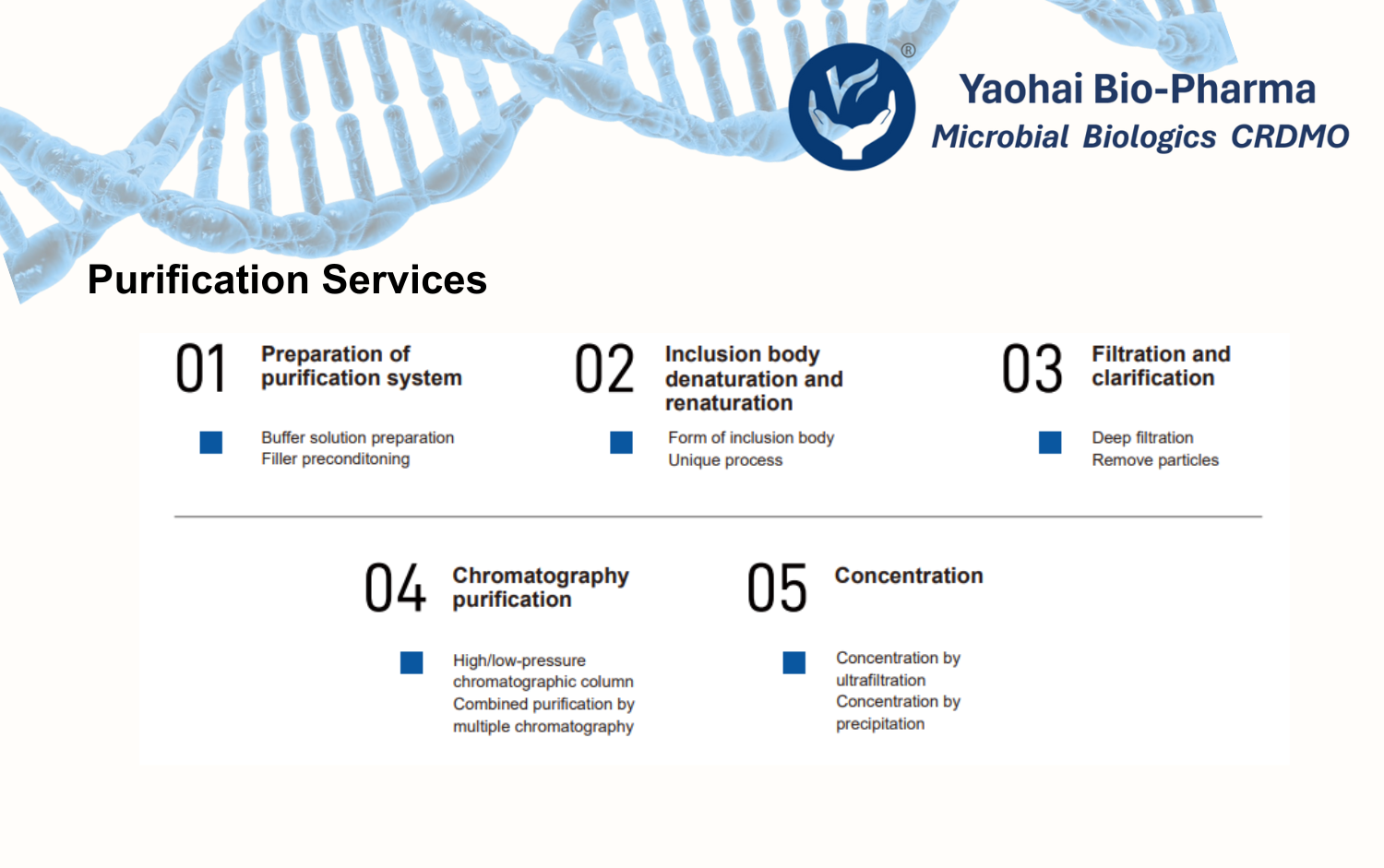
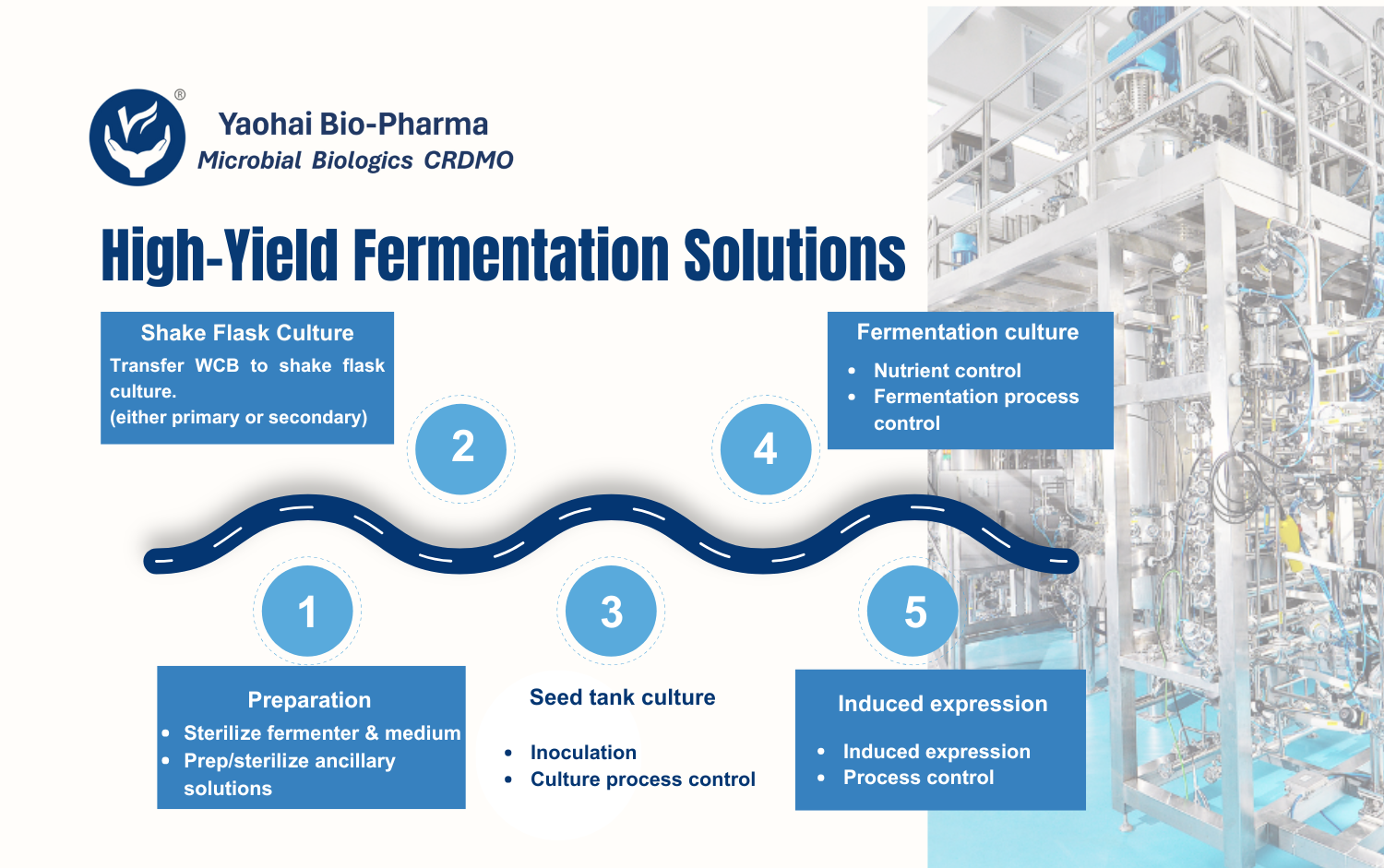
Yaohai Bio-Pharma has more than 15 years of experience in microbial cell line development and cell bank construction. Microbial cell line development and high-yield strain selection are the foundation for master cell bank (MCB) development. Their purpose is to ensure strains are high-yielding and genetically stable. Besides, strains would maintain excellent passage and storage stability. As a result, these steps are critical for reliable and robust biopharmaceutical manufacturing.
According to the pharmacopeia guidelines, master and working cell banks must be fully characterized. This includes their origin, genetic background, and construction process, along with documentation of recombinant strain lineage and biological properties. Meeting these requirements is essential for regulatory compliance and successful downstream production.
Yaohai Bio-Pharma: Comprehensive Strain Development Solutions
Building on our E. coli and yeast expression platforms, Yaohai Bio-Pharma offers full services for strain development, high-yield strain screening, and standardized material characterization. In addition, we provide plasmid design and host cell verification, along with full traceability. We also issue certified Certificates of Analysis (CoA), ensuring quality and regulatory compliance throughout the process.
Our E. coli expression platform uses the widely adopted pET vector series along with common host strains such as DH5α, TOP10, BL21(DE3), BL21 Star(DE3), and BL21 AI. This combination allows flexible optimization for soluble, inclusion body, or secretory protein expression depending on project requirements. Consequently, clients benefit from high-expression, stable, and predictable recombinant protein production.
Yeast Expression System
For Pichia pastoris, we utilize vector systems including pPIC9K, pPICZa, and Pinka-HC, together with host strains such as SMD1168H, X-33, GS115, and PichiaPink strain 1–4. These systems support both intracellular and secreted recombinant protein expression. Moreover, our yeast platform allows flexible process design to meet diverse project needs.
Key Service Highlights
Multi-Host Transformation Platforms
E. coli: DH5α, TOP10, Trans10, and BL21-derived strains
Yeast: SMD1168H, X-33, GS115, and PichiaPink strain 1–4
Clear host lineage with full CoA documentation
Patent-expired hosts prioritized to reduce IP risks
Regulatory-Compliant Selection Systems
Antibiotic-free or regulatory-guided selection markers available
Fully aligned with FDA, EMA, and NMPA expectations
High-Throughput Clone Screening
Supports screening of 100+ clones per run
Rapidly identifies high-expression, stable-producing strains
Robust Documentation and Traceability
Comprehensive construction records ensure full traceability
Fully meets global regulatory requirements for recombinant cell line documentation
By combining advanced platforms with strict regulatory compliance, Yaohai Bio-Pharma helps clients accelerate microbial biologics development while ensuring reproducible, high-quality results.
About Yaohai Bio-Pharma
Yaohai Bio-Pharma is a CRDMO with extensive expertise in microbial expression systems. We focus on recombinant proteins, nucleic acid drugs, nanobodies, plasmid DNA, and innovative vaccines. From R&D to commercial manufacturing, we offer full-lifecycle solutions. Our mission is to build an open, integrated CRO/CDMO/MAH platform that supports global partners from discovery to market.