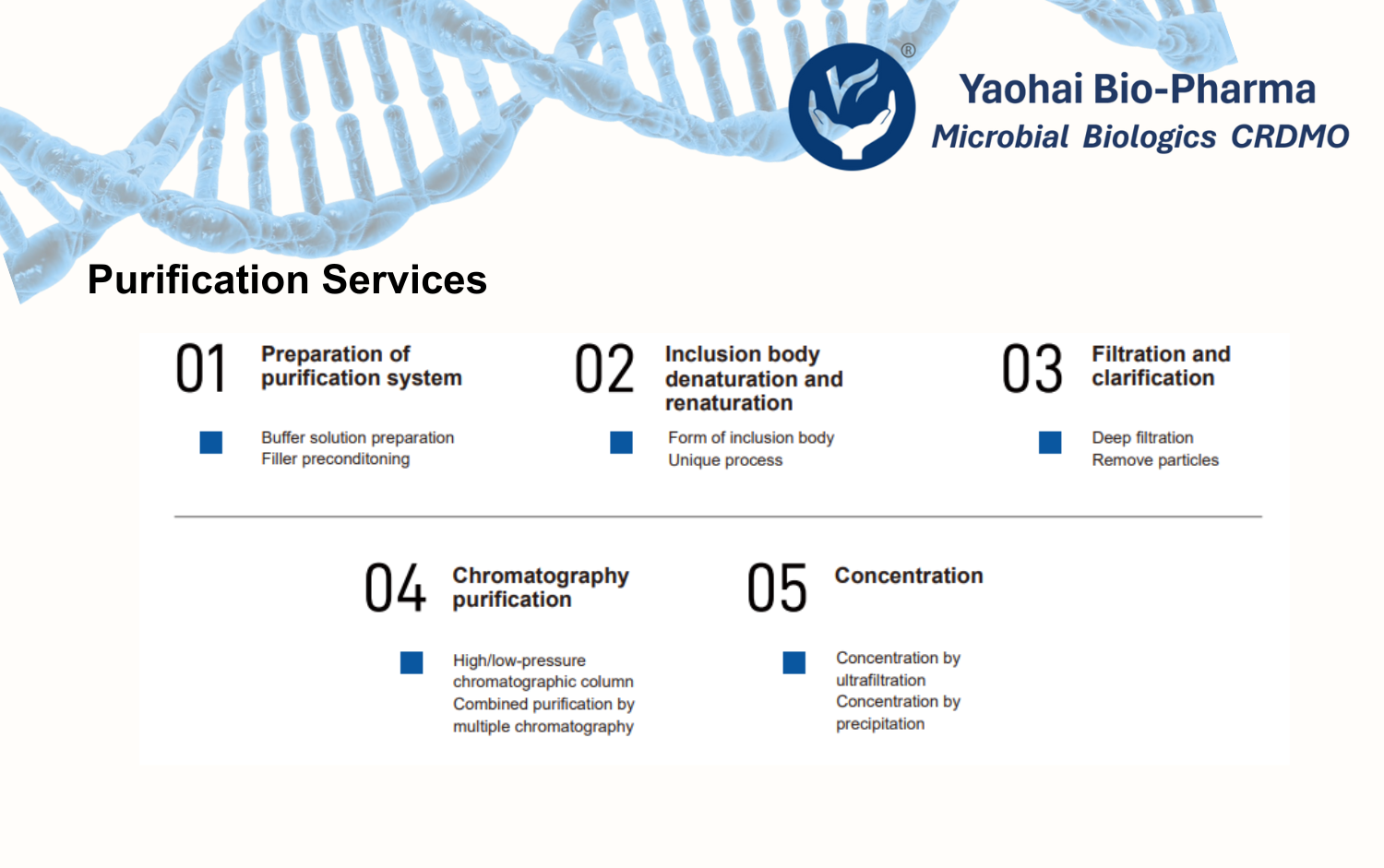
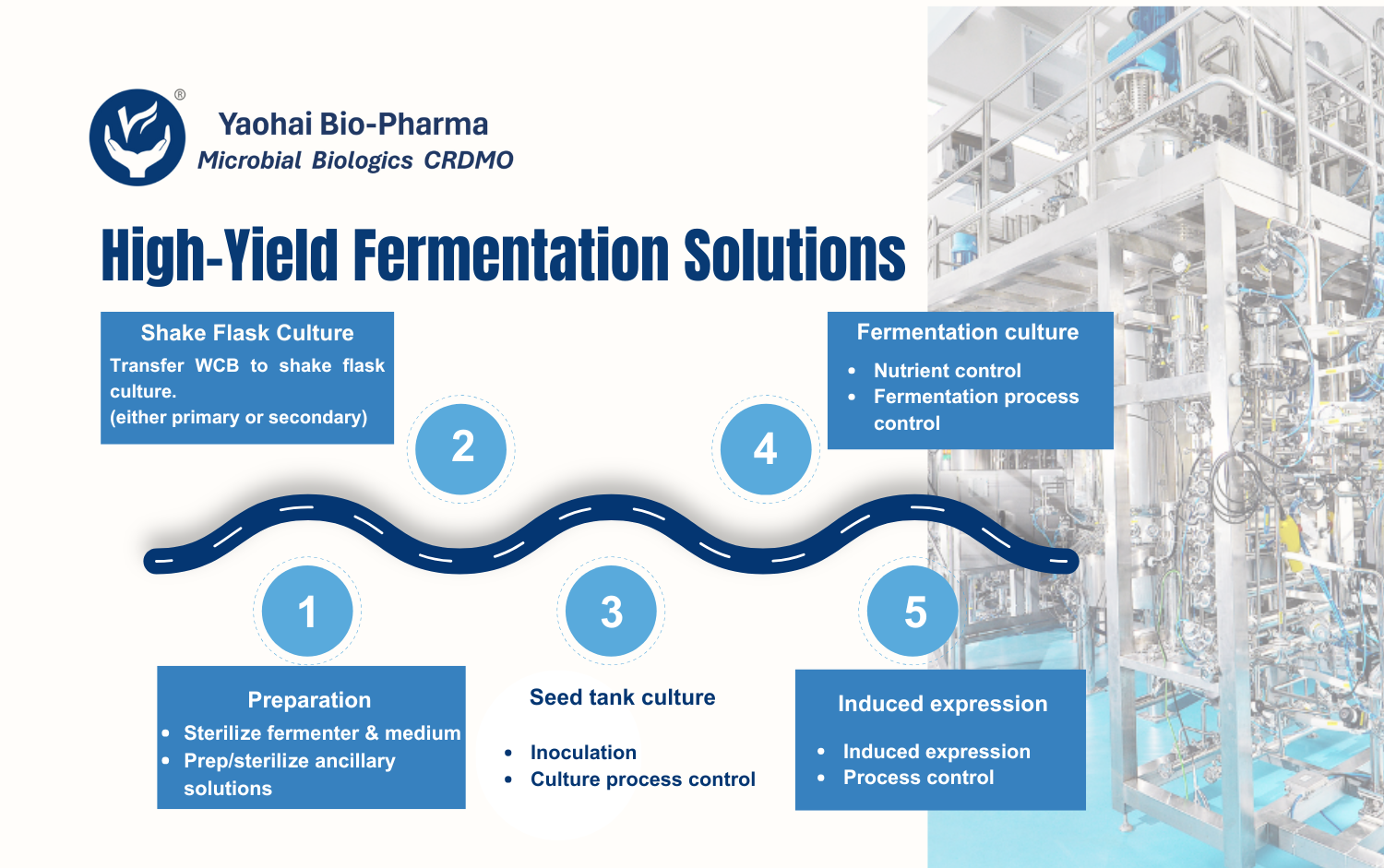
Yaohai Bio-Pharma’s GMP-compliant facilities meet the regulatory standards of the NMPA, FDA, and EMA. We offer flexible bioreactor capacities ranging from 50–100 L to 2000 L, accommodating diverse production needs across all phases of drug development.
Service Content:
- GMP Cell Banking Services
⚬ Fully established under GMP compliance
- Clinical Trial Material Manufacturing
⚬ GMP production for Phase I–III clinical trials
- Process Optimization & Scale-Up
⚬ From pilot-scale development to commercial-scale manufacturing
- Commercial Manufacturing
⚬ Comprehensive manufacturing solutions for MAH license holders
- IND-Enabling GMP Batches
⚬ GMP batch production for regulatory submissions
- Reference Standard Preparation
⚬ Preparation of pharmacopoeia-compliant reference materials
Service Highlights:
- Commercial-Scale Production Capability
⚬ Production of various specifications of drug substance at different scales (50L-100L, 200L, 500L, 1000L and 2000L)
- Compliance Assurance
⚬ Comply with NMPA, FDA, and EMA requirements
⚬ Comprehensive quality management system
⚬ Experienced quality management team
- Proven Technical Experience
⚬ Comprehensive technology transfer process and risk management system
- Powerful Data Management
⚬ Over 80% of the production line is under intelligent operation