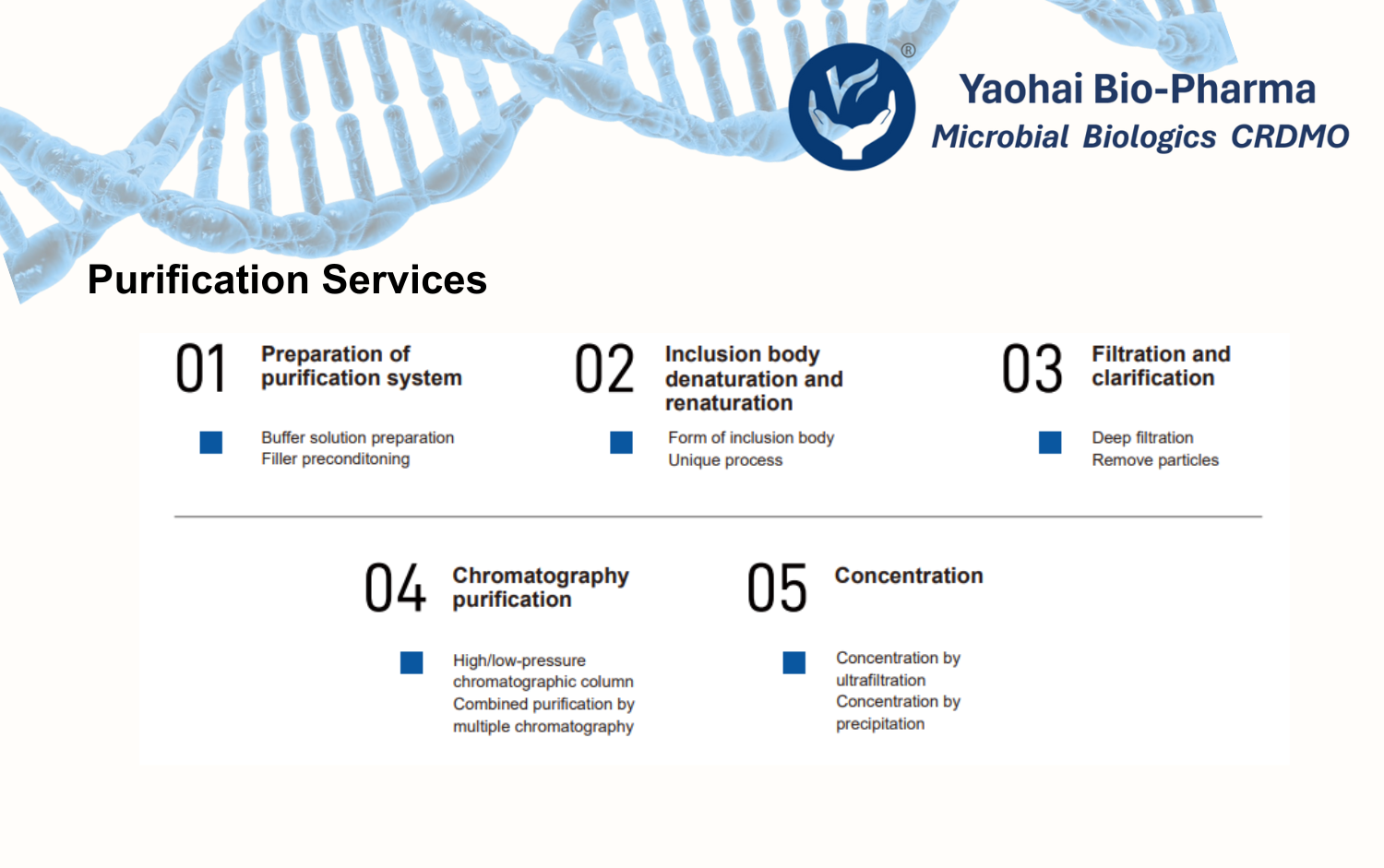
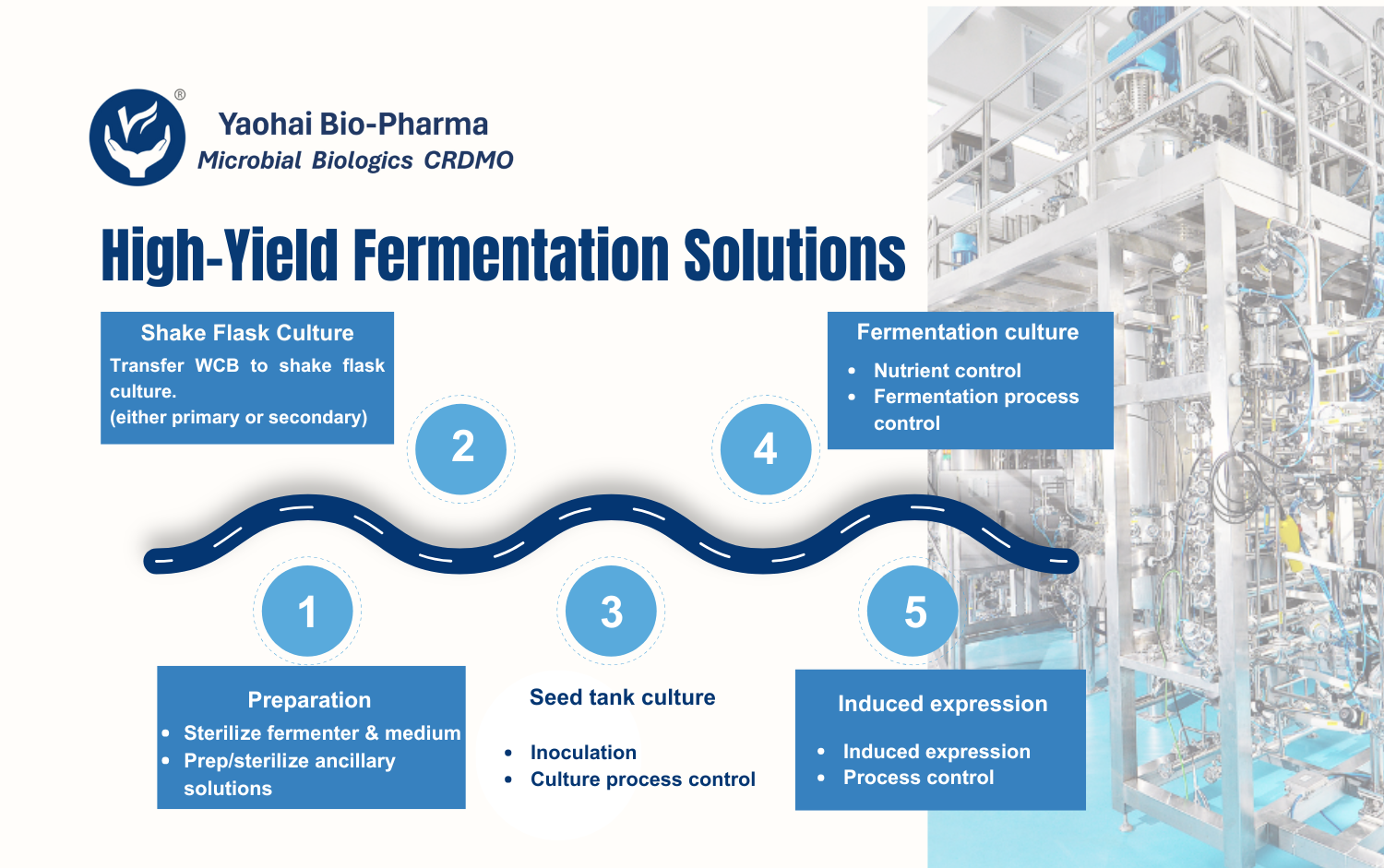
Yaohai Bio-Pharma operates a 2,000 m² GMP-compliant sterile drug product (DP) manufacturing facility equipped with an automated production line integrating vial washing, depyrogenation, filling, lyophilization, and capping.
The facility meets NMPA, EMA, and FDA requirements for aseptic production. With extensive experience in sterile biologics, we offer end-to-end manufacturing services from clinical sample production to large-scale commercial filling of vials and pre-filled syringes (PFS).
DP Production Line for Aseptic Liquid and Lyophilized Vial Preparations :
| Filling specification range |
| 1ml-25ml |
| Sterile production line |
| 1. O-rabs system to protect the Grade A environment in the product (and packaging materials) exposure area 2. Fully automatic feeding and discharging system 3. Fully automatic SIP/CIP system of the lyophilized machine 4. Online nitrogen charging system for nitrogen-filled protection 5. PMS online monitoring system |
| Filing Precision |
| High filling precision: (filling medium: water for injection) ± 0.25% |
| Filling speed |
| The maximum production speed is 300 vials/min, and the maximum lyophilization batch is 37800 vials /batch, calculated based on the 2ml vial |
DP Production Line for Aseptic Pre-filled Syringe/Cartridge Preparations :
| Filling Specifications |
| Pre-filled syringe: 1ml and 3ml, cartridge: 3ml |
| Filling Process Plunger pumps and peristaltic pumps, with a dual-pump system to meet various filling process requirements for different drug solutions |
| Filing Precision |
| For 0.2–0.5 mL: ±3% For 0.5–3 mL: ±2% |
| Aseptic Filling |
| 1. Various drug liquid filling methods (normal filling, nitrogen filling, vacuum filling) 2. Vacuum stoppering method, applied to various rubber stoppers process 3. O-rabs system to protect the Grade A environment in the product (and packaging materials) exposure area 4. PMS online monitoring system to facilitate the real-time monitoring of the production environment and quick detection of environmental abnormalities
|
Service Highlights:
- Aseptic Fill & Finish Capabilities
- Vials (Liquid): 10 million units/year
- Vials (Lyophilized): 5 million units/year
- Pre-filled syringe (PFS)/cartridges: 10 million units/year
- Manufacturing Capability Scope
- IND-enabling, Clinical Phase I/II/III, and Commercial Production
- Biologic Portfolio
- Recombinant protein, peptide, plasmid DNA, nano-bodies, vaccines, and other mainstream biologics