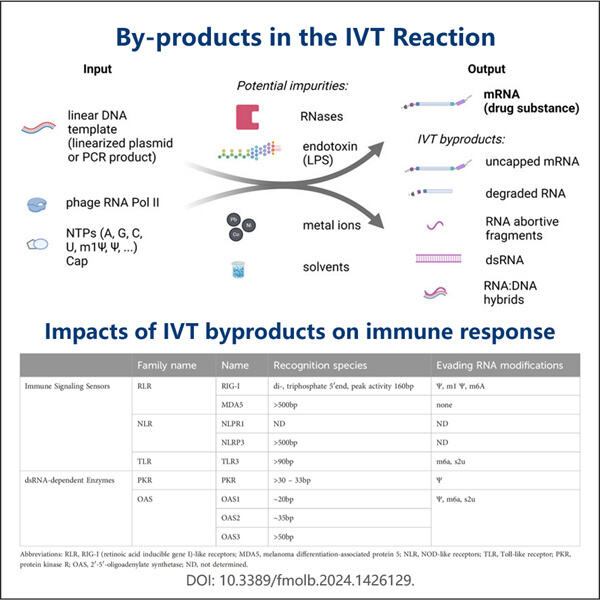
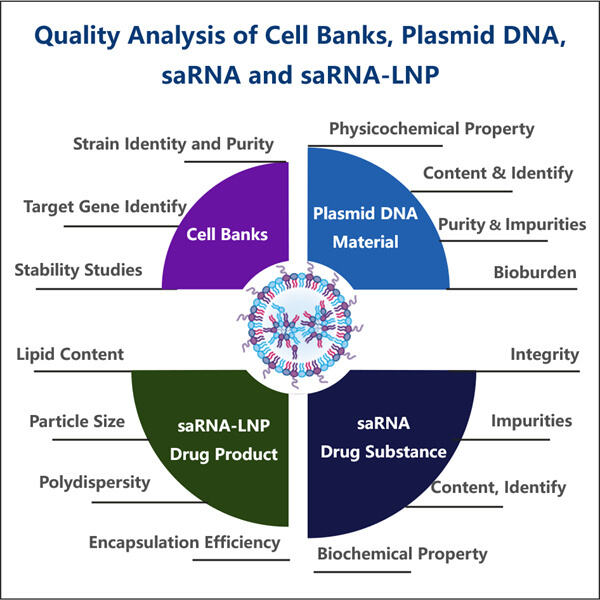
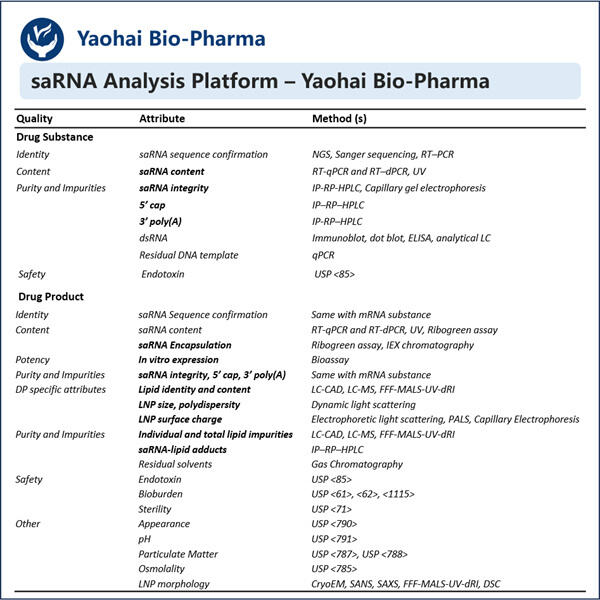
Ein solches Unternehmen, das ein außergewöhnlich einzigartiges Produkt namens saRNA herstellt, heißt Yaohai. saRNA – small activating RNA. Diese Yaohai Produkte hat eine lebenswichtige Molekülstruktur, die uns helfen lässt, zu gehen und unser Blut zu verdicken. Wir haben auch Gene, die im Wesentlichen Anweisungen sind, die unserem Körper sagen, wie er wächst und funktioniert. Angesichts der Macht dieses Prozesses ist es von entscheidender Bedeutung, sicherzustellen, dass die als Medizin oder experimentelles Werkzeug verwendete saRNA hohe Qualität aufweist.
Dies ist einer der Hauptgründe, warum es so wichtig ist, die Qualität von saRNA zu testen, da sie sehr spezifisch sein müssen. Mit anderen Worten, wir sollten Kontrolle über die Gene haben, auf die sich saRNAs auswirken. Es sollte die anderen Gene, die wir nicht modifizieren möchten, nicht stören. Wenn es sich nicht an das richtige Gen bindet, kann das Haushaltungsprotein potenziell erhebliche Schäden verursachen und unabsichtlich den Organismus verletzen, da es zufällige Gene beeinflussen würde. Dies Yaohai saRNA-LNP-Encapsulation-Protokoll kann unerwünschte Nebenwirkungen oder Verhaltensweisen verursachen, daher ist eine ordnungsgemäße Kontrolle essenziell.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN