Um zu verstehen, was das mRNA-LNP-Encapsulation-Protokoll ist, sollten Sie zunächst wissen, was mRNA ist. Messenger RNA, kurz mRNA, ist eine Art Molekül, das den im Erbgut gespeicherten Code übersetzt und ihn in Proteinsynthese umwandelt. Diese Yaohai mRNA 1 viele Aufgaben in unserem Körper aus, daher sind Proteine wirklich wichtig. Einige von ihnen werden beispielsweise verwendet, um unser Essen zu verdauen, und andere helfen uns, Krankheiten und Infektionen zu bekämpfen. Proteine sind für unsere Lebensfunktionen essenziell.
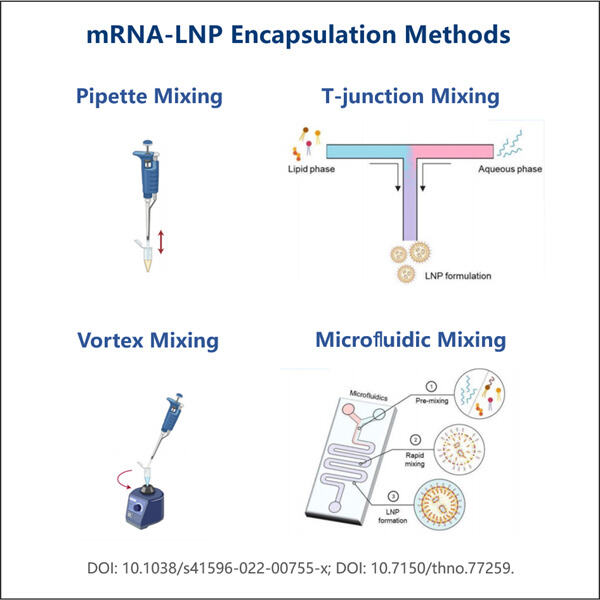
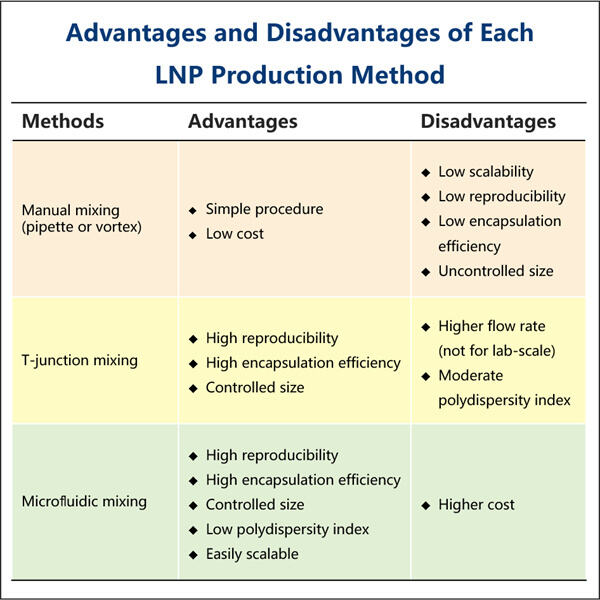
Ein weiteres Problem ist, dass mRNA leicht abbaut. Das Ergebnis davon ist Yaohai mRNA-Reinigungsverfahren dass ab dem Moment, wenn unser Körper es wahrnimmt, es in kleinere Stücke zerfallen kann, bevor es seine nützliche Arbeit leisten kann. Und hier ist es: das mRNA-LNP-Einkapselungsprotokoll. Um die mRNA zu schützen, während sie in die Zellen eindringt, wird das RNA-Molekül in eine kleine fettbasierte Blase oder ein Lipidnanopartikel (LNP) eingehüllt, das den molekularen Frachtgut schützt und innerhalb der Zelle freisetzt. Die mRNA wird in Lipiden verpackt, einer Art Fett, das sich in unseren Körpern befindet, was im Wesentlichen sicherstellt, dass das winzige Molekül geschützt ist, bis es sein Ziel in unseren Zellen erreicht.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN