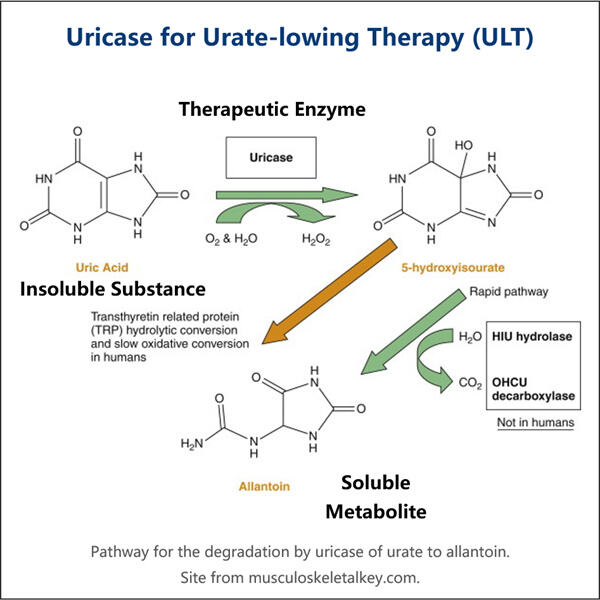
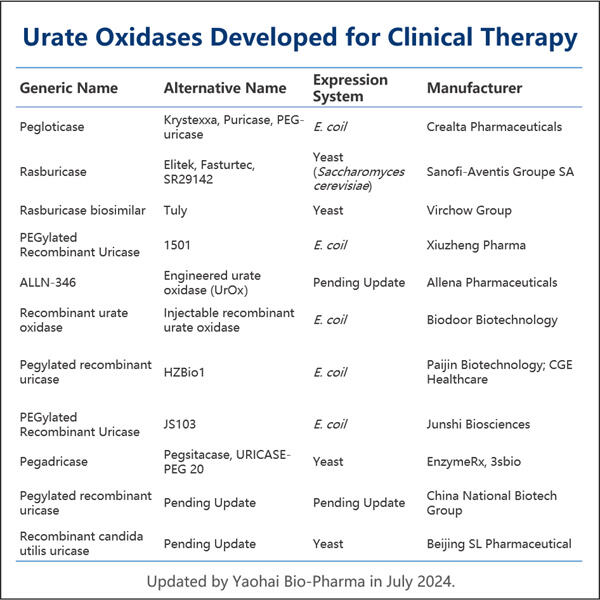
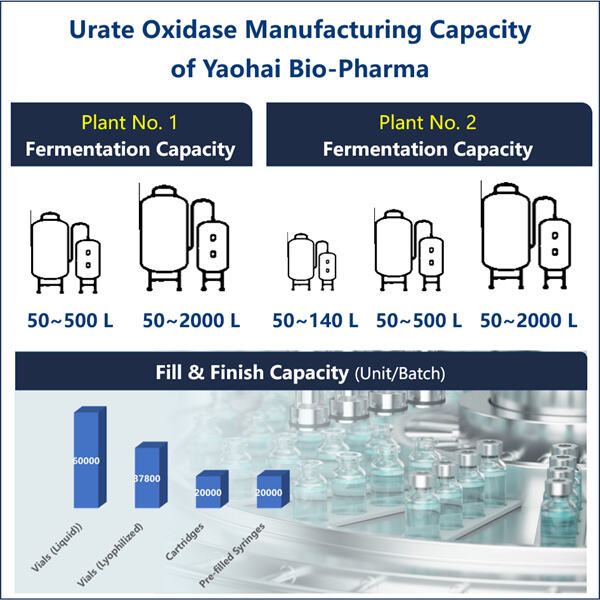
In diesem Handbuch werden wir detailliert darlegen, wie Yaohai ein solches Protein herstellt: rekombinantes Uratoxidase. Eine der Bedingungen, bei denen dieses Protein Linderung bringen kann, ist Gicht - eine schmerzhafte Erkrankung, die die Gelenke betrifft. Wir werden die verschiedenen Schritte behandeln, die notwendig sind, um dieses Schlüsselprotein herzustellen, sowie die Schwierigkeiten und das zukünftige Potenzial in diesem Bereich.
Wissenschaftler müssen den bestimmten Bereich der DNS finden, der die Anweisungen zur Produktion von Uratoxidase enthält, um diesen Prozess zu starten. Zunächst suchen sie nach dem Gen, das dieses Protein kodiert, und um es zu finden, müssen sie in der DNS bei Menschen oder Tieren suchen. Nachdem sie dieses Gen identifiziert haben, entfernen sie es und platzieren es in ein kreisförmiges Stück DNS namens Plasmid. Das Plasmid ist einzigartig, da es sich selbst replizieren kann. Sobald das Plasmid vorbereitet wurde, nehmen wir dieses Plasmid und setzen es in eine Wirtszelle (eine bestimmte Art von Bakterien oder Hefen). Die Wirtszellen beginnen dann, das Uratoxidase-Protein herzustellen.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN