Identifizierung und Charakterisierung von Proteinen durch Massenspektrometrie
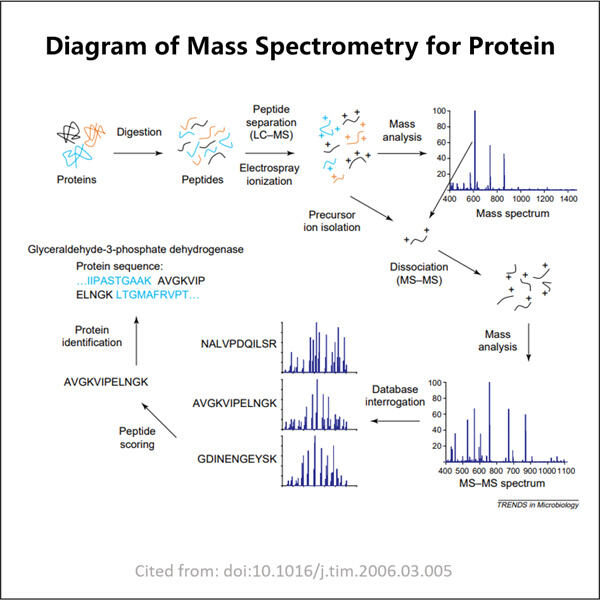
Und nun zu den Proteinen. Proteine sind essenziell für das Leben, da sie helfen, die Struktur unseres Körpers aufzubauen und zu erhalten. Diese bestehen aus kleineren Einheiten, bekannt als Aminosäuren. Aminosäuren können als Bausteine betrachtet werden, und wenn viele von ihnen zusammenwirken, werden sie zu einem Protein. Aminosäuren sind die Bausteine von GMP Anti-PD-1PD-L1 VHH proteinen, daher kann Massenspektrometrie helfen, welche Aminosäuren ein Protein ausmachen und woraus dieses Protein besteht
Wenn Wissenschaftler Proteine mit Massenspektrometrie untersuchen, zerlegen sie das Protein in kleinere Stücke. Sie erreichen dies, indem sie einige spezielle Helfer verwenden, die Enzyme, die als Scheren wirken und das Protein zerschneiden. Nachdem es in kleinere Fragmente aufgeteilt wurde, können Wissenschaftler diese kleineren Fragmente in geladene Teile umwandeln und sie mittels Massenspektrometrie trennen. Anhand des resultierenden Diagramms (wie dem unten gezeigten) können Wissenschaftler dieses Diagramm dann mit bekannten Proteinen vergleichen, um mehr über das individuelle Protein herauszufinden, das sie untersuchen.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN