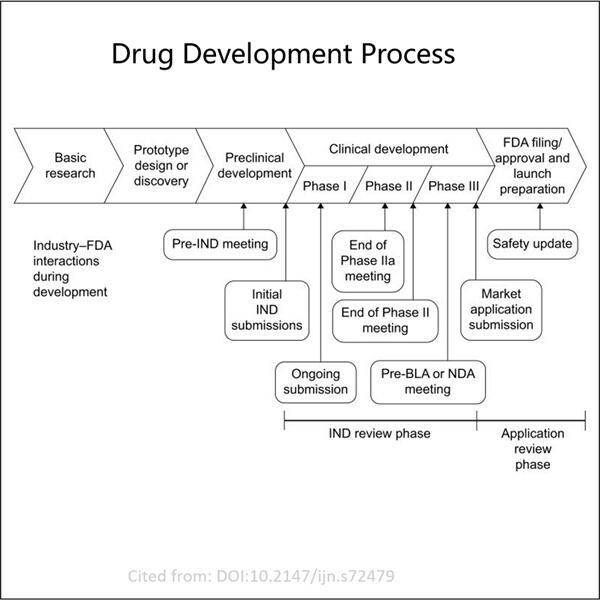
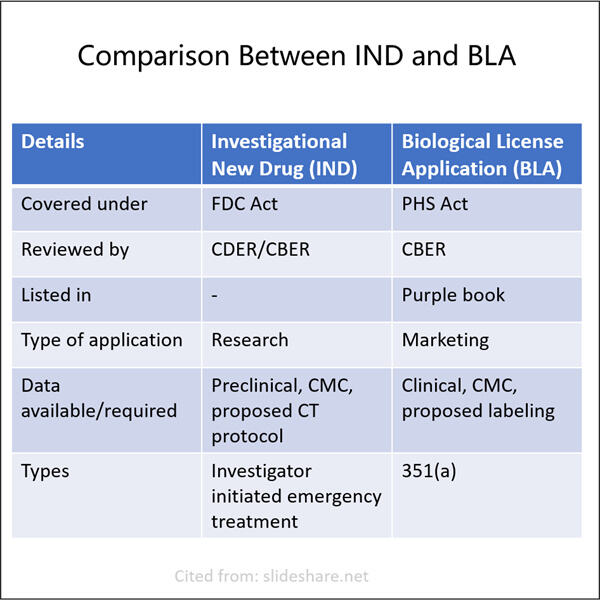
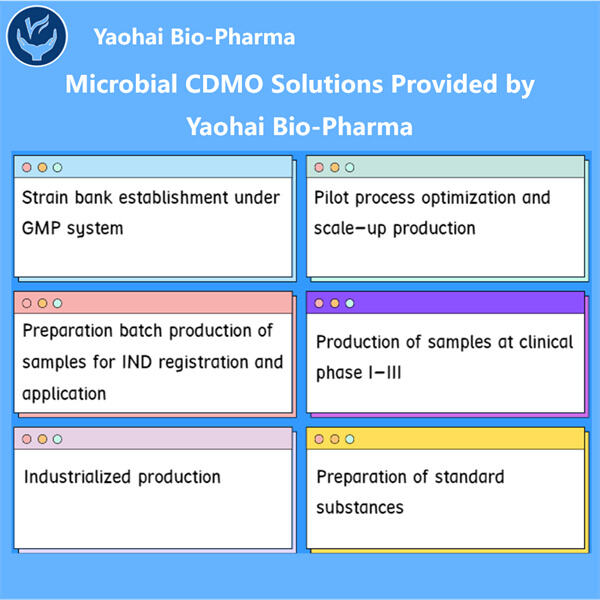
Yaohai kennt sich gut mit Bergmedizin aus. Sie entwickelten zwei Arten wichtiger Anwendungen – IND (Indol) und BLA (Blubber). IND steht für Investigational New Drug und wird verwendet, um die Erlaubnis der US FDA zu erhalten, ein neues Medikament an Menschen zu testen. Ein Biologics License Application (BLA) ist erforderlich, wenn ein Unternehmen biologische Produkte verkaufen möchte. GMP Semaglutide API diese Anträge sind entscheidende Schritte, um sicherzustellen, dass neue Medikamente sicher und wirksam für Menschen sind. In diesem Artikel werden wir diskutieren, wie man diese beiden Arten von Anträgen einreicht und ihre Unterschiede sowie Ähnlichkeiten.
Das Unternehmen, das einen Antrag auf ein IND stellen möchte, muss zunächst eine spezielle Anfrage an die Food and Drug Administration (FDA) richten. Dies ist ziemlich notwendig, da diese Anfrage alle wesentlichen Details zu diesem neuesten Medikament enthalten wird. Die Anfrage wird nun vor der FDA zur Prüfung liegen, während das Unternehmen die Erlaubnis sucht, mit den Tests des Medikaments an Menschen zu beginnen. Dieser Teil des Prozesses dauert normalerweise bis zu 30 Tage. Zweitens, sobald diese fehlenden Informationen eingereicht und von der FDA akzeptiert wurden – falls sie genehmigt wird – kann das Unternehmen mit den Tests an Menschen beginnen, was einen großen Schritt in der Entwicklung eines neuen Medikaments darstellt.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN