Anpassung, Effizienz & Kosteneffektivität
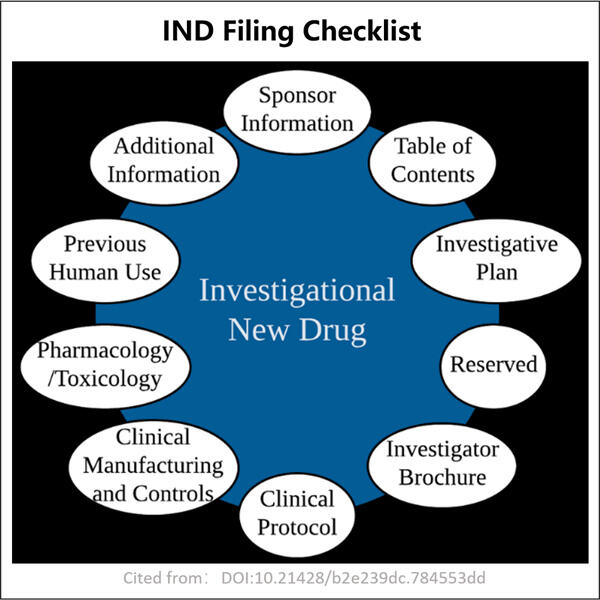
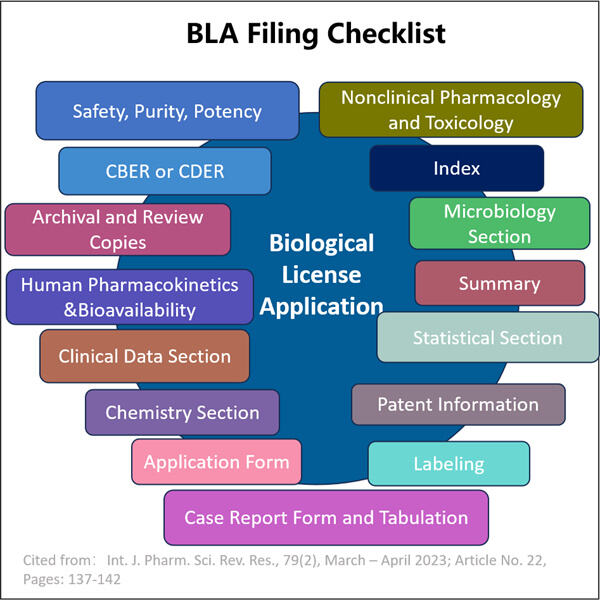
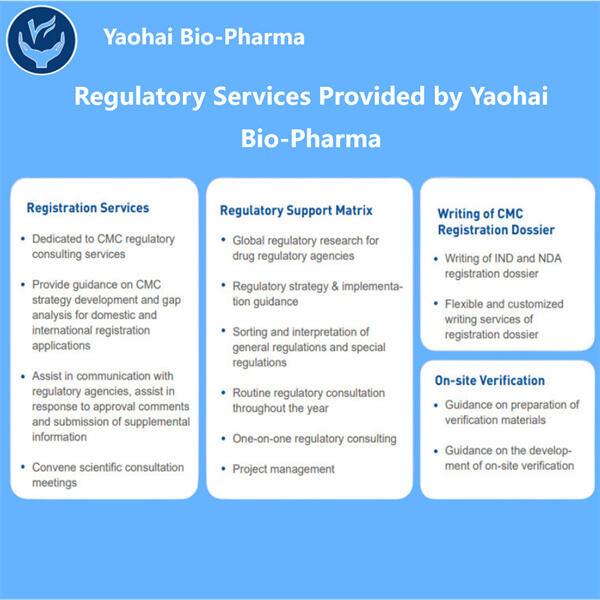
Yaohai Bio-Pharma hat Erfahrung in der Entwicklung von aus Mikroorganismen stammenden Biologika. Wir bieten maßgeschneiderte Forschungs- und Entwicklungsleistungen sowie Produktionslösungen, wobei wir dafür sorgen, dass keine Risiken bestehen. Wir haben an verschiedenen Modalitäten gearbeitet, wie substanzbasierten rekombinanten Impfstoffen, IND- und BLA-Füllchecklisten, Zytokinen, Wachstumsfaktoren, Single-Domain-Antikörpern, Enzymen, Plasmid-DNA, mRNA und anderen. Wir sind Experten in einer Vielzahl von Mikroorganismen, einschließlich Hefextrazellen- und intrazellulärer Sekretion (Erträge bis zu 15g/L) sowie bakterieller intrazellulärer Löslichkeit und Inklusionskörper (Erträge bis zu 10g/L). Außerdem haben wir die BSL-2-Gärungsplattform entwickelt, um bakterielle Impfstoffe herzustellen. Wir haben eine bewährte Methode zur Optimierung von Produktionsprozessen, was die Erträge erhöht und die Kosten senkt. Mit einem hoch effizienten Technologie-Team stellen wir sicher, dass Projekte pünktlich und qualitativ hochwertig abgeschlossen werden und Ihre Produkte schneller auf den Markt kommen.

 EN
EN
 AR
AR
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 SL
SL
 UK
UK
 VI
VI
 ET
ET
 HU
HU
 TH
TH
 TR
TR
 FA
FA
 AF
AF
 MS
MS
 BE
BE
 MK
MK
 UR
UR
 BN
BN