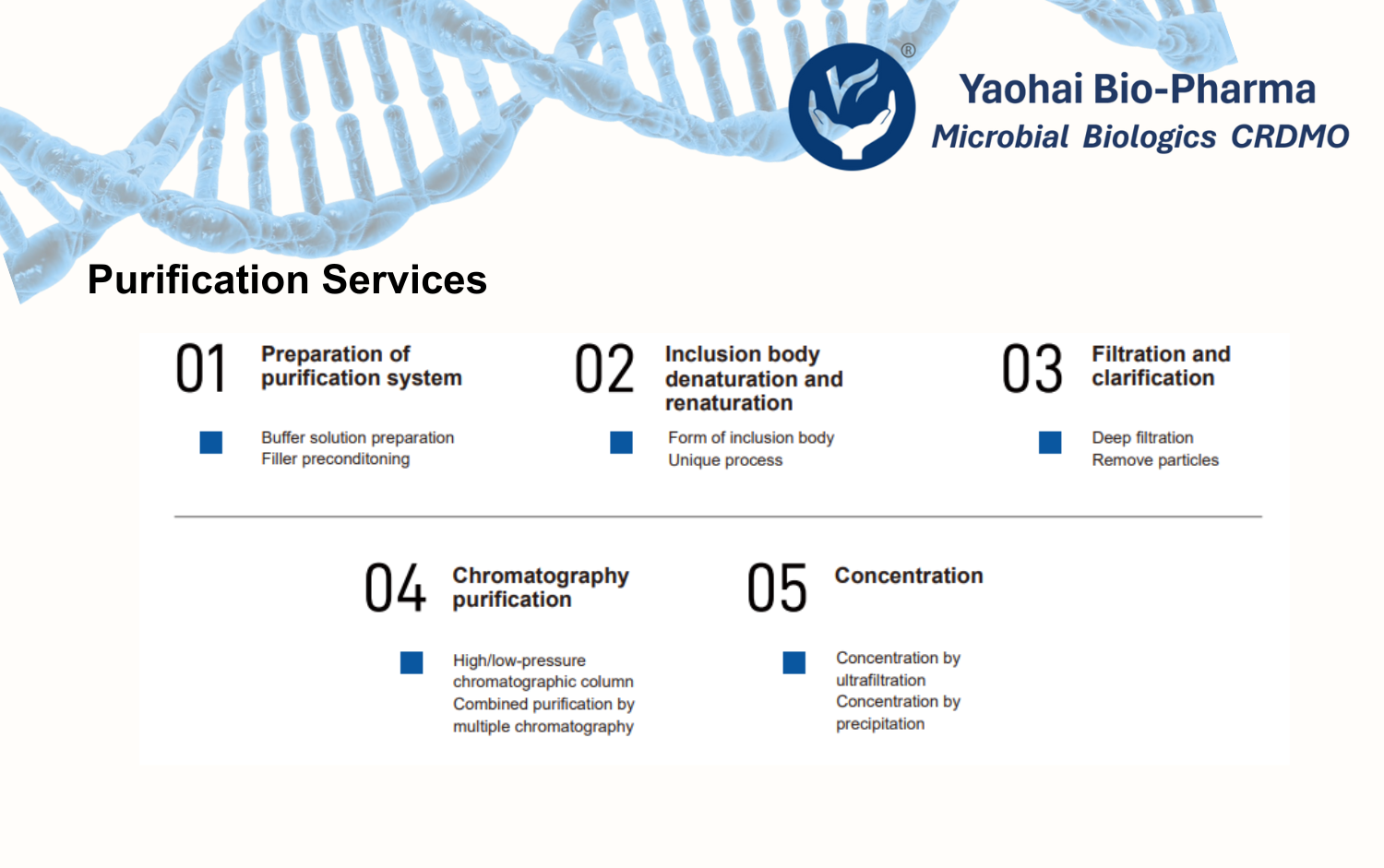
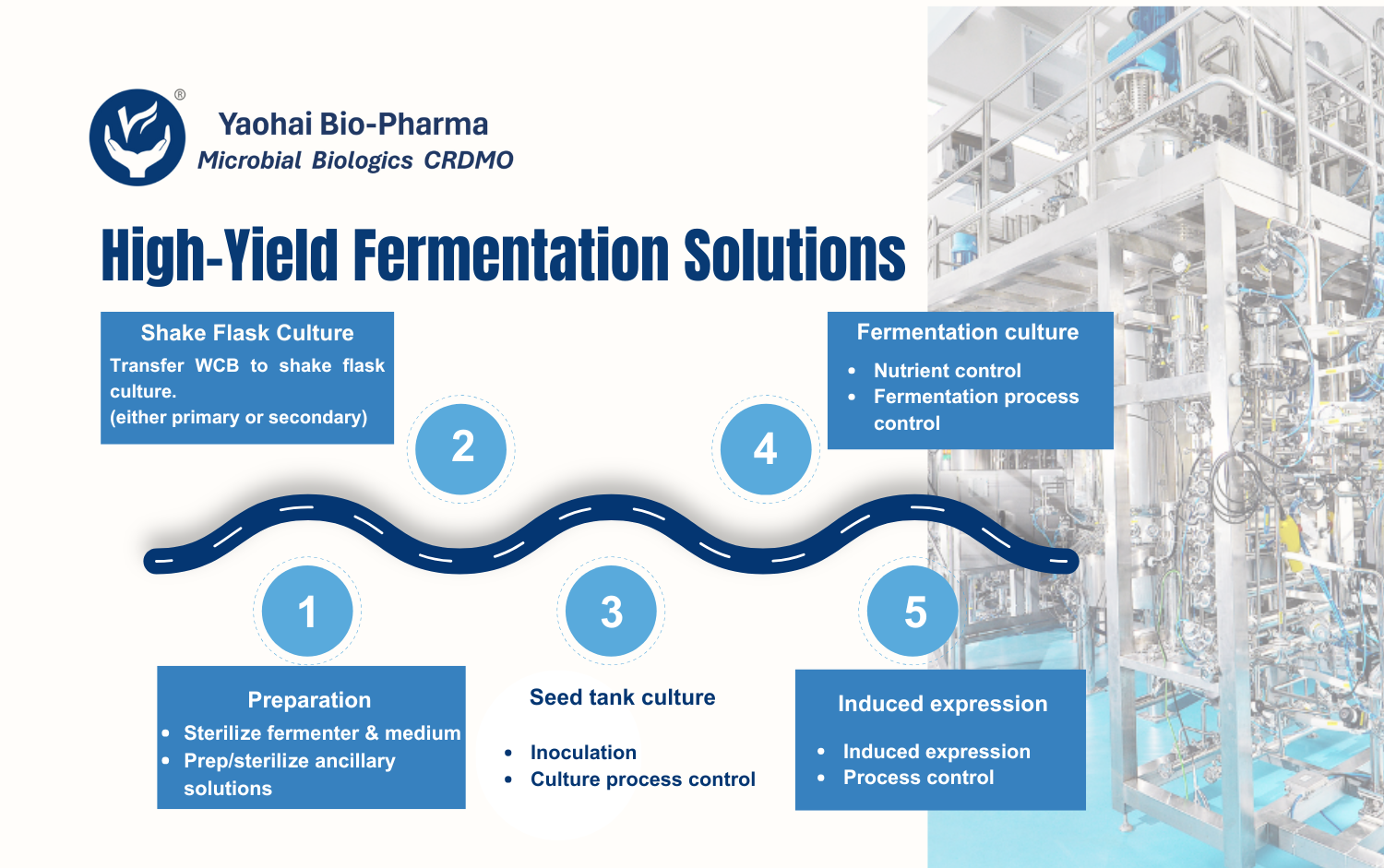
Yaohai Bio-Pharma’s flexible process development platform supports various R&D stages, with QbD principles integrated throughout. Backed by technical expertise and project experience, we help clients achieve efficient, cost-effective GMP production.
Service Content:
- Media screening and optimization
- Fermentation process development and optimization
- Scale-up development across bioreactor platforms: 7L, 30L/50L, 100L, 200L, 500L, 1000L, 2000L
- Regulatory support (FDA/EMA/NMPA compliance)
Service Highlights:
- Expert process development team support
- Diverse project execution experience
- Robust platform-based bioprocess workflows
- Agile development strategy powered by QbD and DoE methodologies