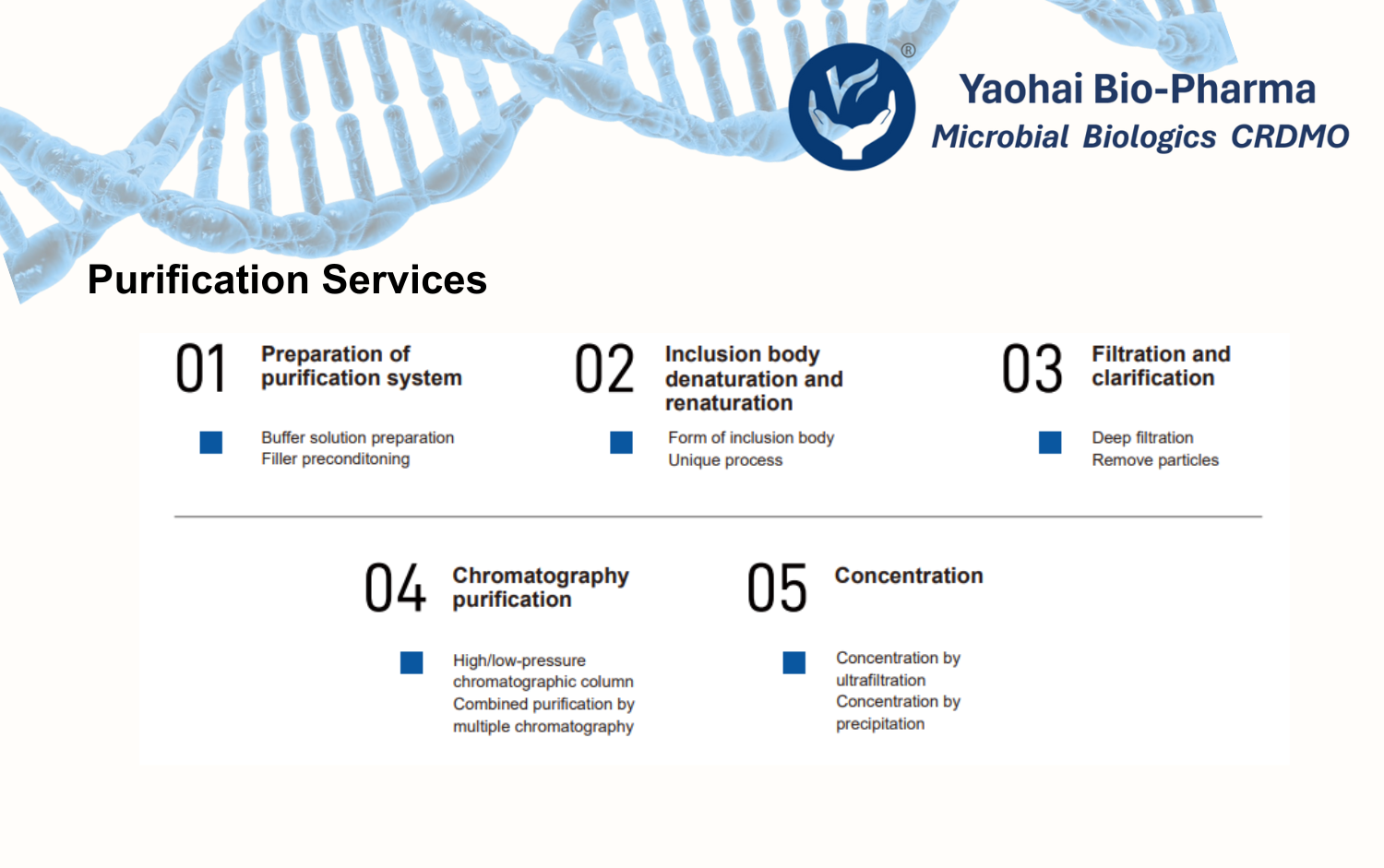
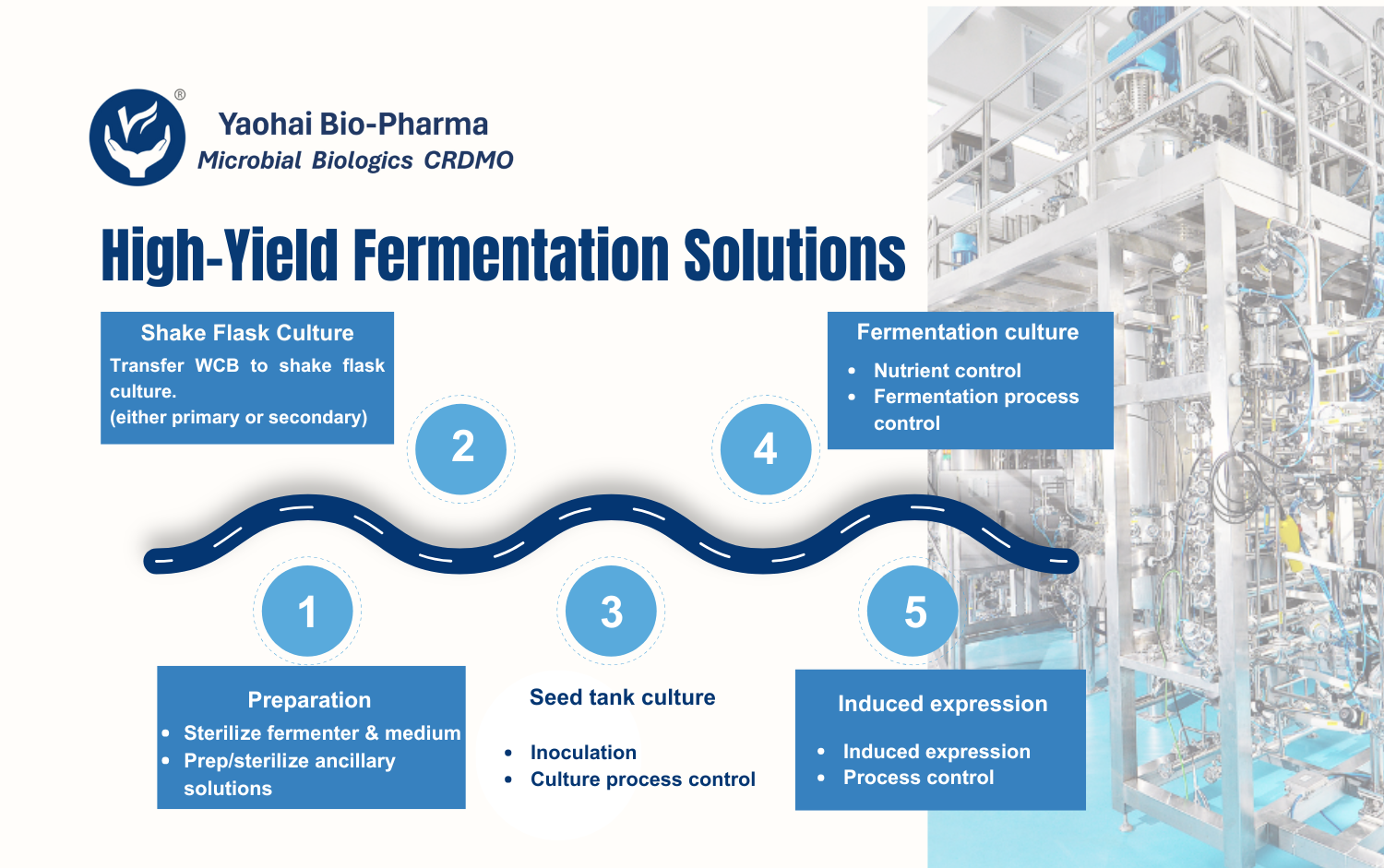
Yaohai Bio-Pharma’s GMP facility features dedicated suites for PCB, MCB, and WCB preparation and storage, with comprehensive support for stability studies and passage. From gene synthesis to GMP-compliant microbial cell banking, we provide reliable and end-to-end solutions.
Service Content:
- Codon Optimization
- Recombinant Plasmid Construction
- Recombinant Strain Construction
- Primary Cell Bank (PCB) Development
- Master Cell Bank (MCB) & Working Cell Bank (WCB) Development, Characterization, and Stability Studies
- Regulatory Submission Services
Service Highlights:
- Traceable Microbial Strains
⚬ All strains are sourced from authorized official institutions with full traceability. - Regulatory-Compliant Cell Banking
⚬ We provide cell banks meeting FDA filing requirements. - Cell Bank Qualification & Release
⚬ Comprehensive testing and release services compliant with FDA standards, ensuring readiness for domestic and international regulatory submissions.
- IND-Ready Cell Banks
⚬ Generation of secondary cell banks suitable for Investigational New Drug (IND) applications.
- Chinese Pharmacopoeia (ChP) Compliance
⚬ Rigorous testing and release of all cell banks according to the Global Pharmacopoeia standards, guaranteeing sterility and storage stability.
- Dedicated Cell Banking Facility
⚬ Independent facility designed to eliminate cross-contamination risks.
- GMP-Compliant MCB/WCB Banking
⚬ Secure and reliable development of Master and Working Cell Banks under GMP standards, governed by standardized cell banking procedures.
- Experience & Expertise
⚬ Profound experience with microbial expression systems, specializing in E. coli and yeast platforms to meet diverse, customized research service needs.