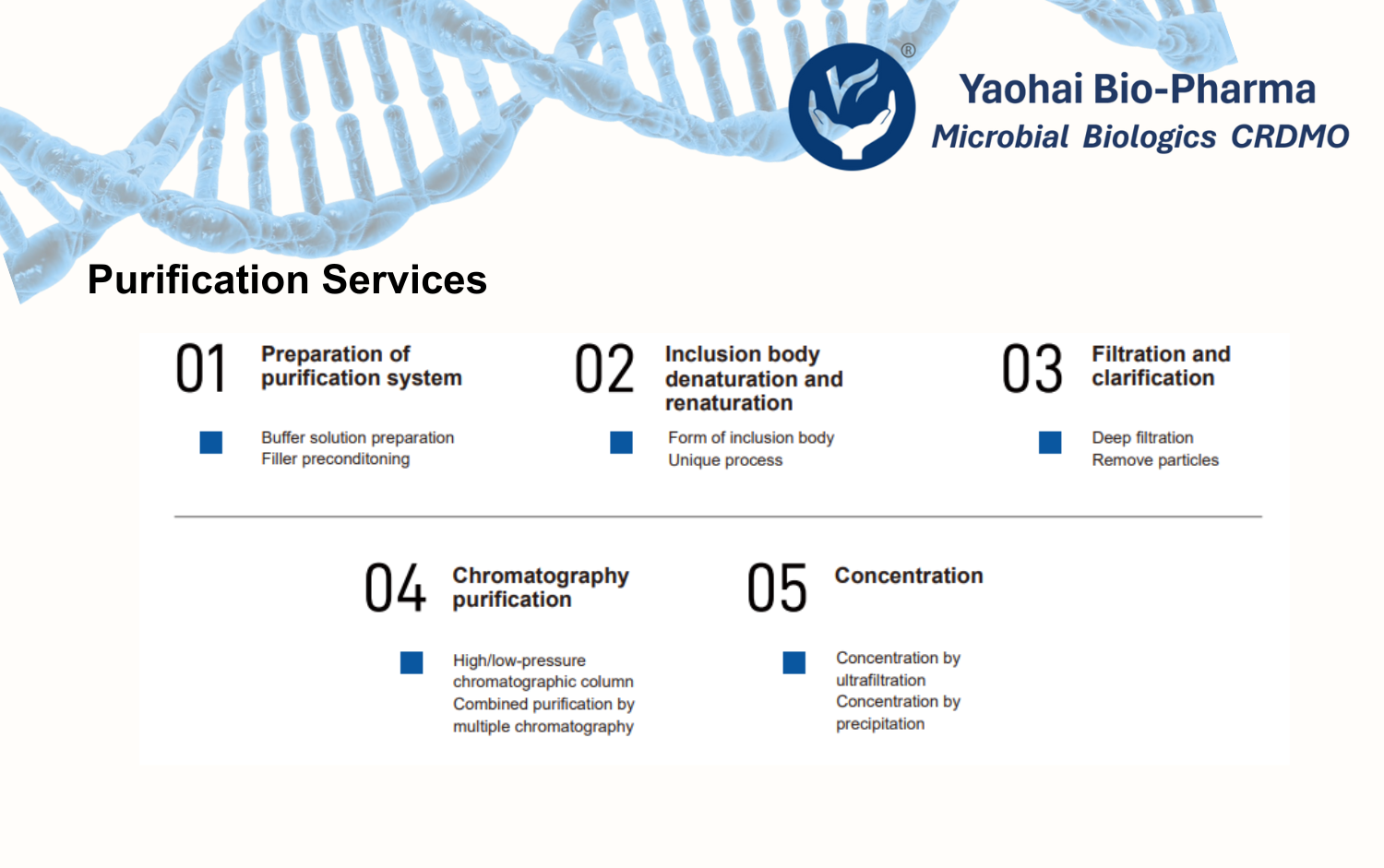
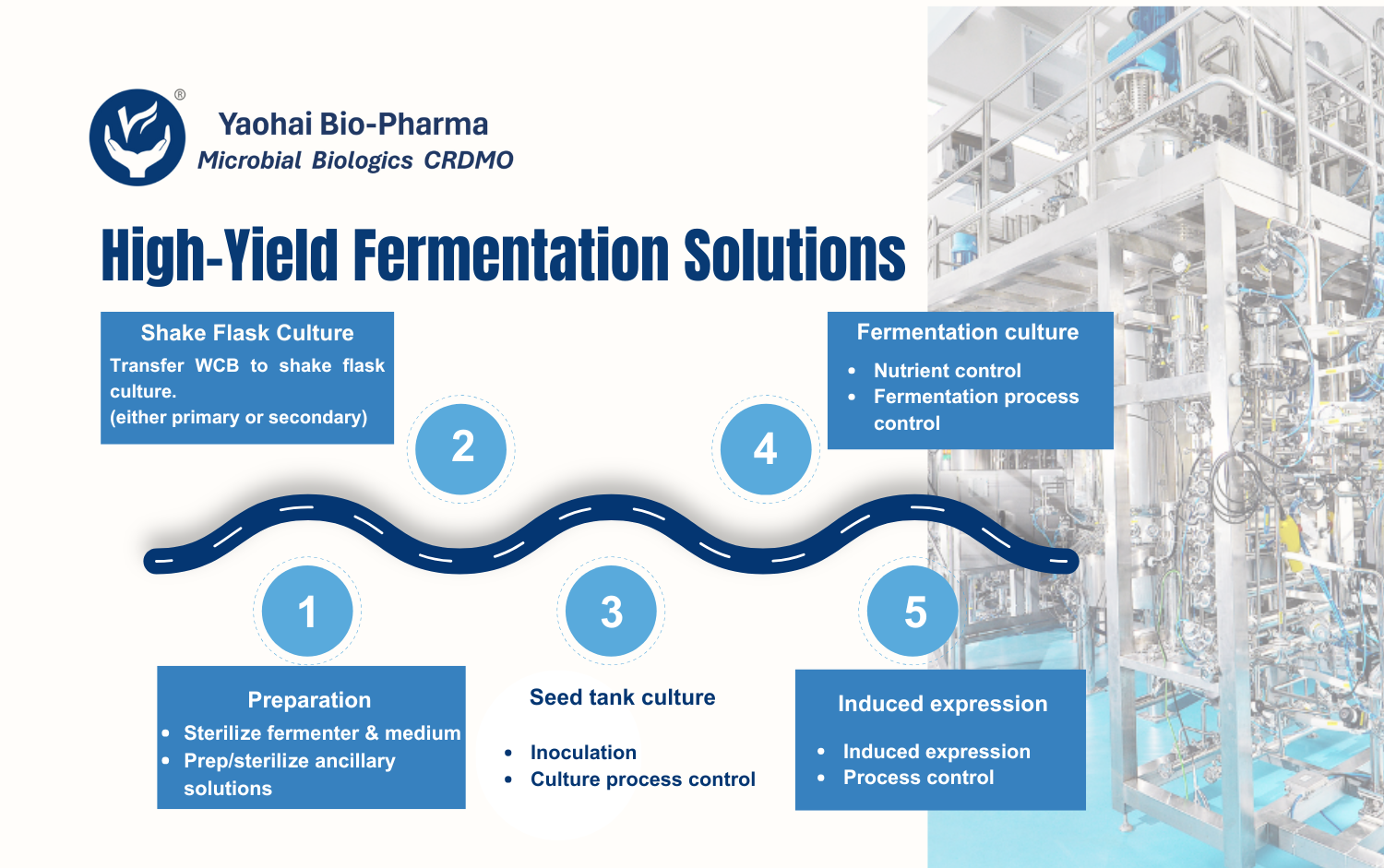
Yaohai Bio-Pharma’s quality research team has deep expertise in analyzing biologics, including recombinant proteins, plasmids, cytokines, and vaccines. Supported by a robust analytical platform and advanced instrumentation, we provide quality analysis services such as method development and validation, stability studies, and reference standard establishment. Our method transfer processes strictly comply with ICH, WHO, and major pharmacopoeial regulations. Our solutions span the entire development cycle, from preclinical research to GMP manufacturing.
Service Content:
- Original strain testing and stability study
- Process development sample analysis
- Identification, assay, purity, activity, impurities, etc.
- Analytical method management
- Analytical method development/optimization: biochemical activity; purity, excipient content, process residues (HPLC), etc.
- Method qualification/validation
- Method transfer
- Specification establishment (including strains, drug substance, drug products, etc.)
- Recombinant protein
- Plasmid DNA
- Vaccine
- Stability pre-study
- Preparation, standardization, and characterization of reference standards
Service Highlights:
Yaohai Bio-Pharma is equipped with advanced analytical instruments, including HPLC, UHPLC, CE, fluorescent PCR, fluorometer (Qubit), multimode microplate reader, gel imaging system, nanoparticle size analyzer, UV spectrophotometer, osmometer, and stability chambers. These resources enable us to provide high-quality analytical and testing services to our clients.